|
|||||||||||
|
|
|||||||||||
|
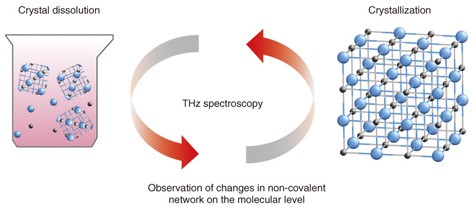
Feature Articles: Cutting-edge Device and Materials Technologies for Creating New Business Vol. 12, No. 4, pp. 39–44, Apr. 2014. https://doi.org/10.53829/ntr201404fa6 Terahertz Spectroscopic Identification of Pharmaceutical Crystals: Cocrystals and Polymorphic FormsAbstractTerahertz radio waves have about the same energy level as the non-covalent bonds between molecules, so they can be used in a spectroscopic technique for identifying differences in molecular networks. We introduce terahertz spectroscopy as a revolutionary technique for visualizing the molecular bonds in various forms of pharmaceutical crystals such as cocrystals and polymorphic forms that are closely related to crystal properties that have not yet been understood. Examples of such properties include solubility and systemic absorption rate. Keywords: terahertz spectroscopic identification, cocrystals and polymorphic forms, pharmaceutical crystals 1. IntroductionThe simple reactions of dissolving a crystal in water and forming a crystal from a solution are very important features of pharmaceutical crystals. Terahertz (THz) spectroscopy can clarify changes in non-covalent bonds of the molecules (Fig. 1), which makes it possible to understand the dynamics of crystal dissolution and crystallization. Pharmaceutical molecules are extracted from solution as pharmaceutical crystals and then formed into pills, tablets, etc. The pharmaceutical crystals must be dissolved in water to be absorbed in the body; otherwise the drug will have no effect. Pharmaceutical crystals that have greater molecular complexity are being developed every year, so the proportion of drugs that do not dissolve easily is presumably also increasing. Although the network of non-covalent bonds on the crystal surface is considered to be closely related to solubility, that network is described by data on hydrogen bonds, van der Waals interactions, or other such interactions between molecules rather than by data on the periodic structure of the crystal obtained by x-ray diffraction measurement. It is currently difficult, therefore, to observe this network directly, and this difficulty is even greater with ultra-fine particles.
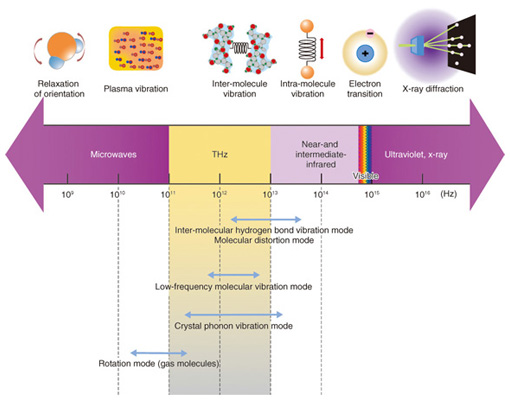
We have approached the problem of elucidating the crystal dissolution mechanism by using THz (THz = 1012 Hz) spectroscopy, which can measure a spectrum in the energy band of molecular interaction. THz light frequency is in between the light and radio frequencies (Fig. 2) and can be used to investigate various molecular properties through resonance, such as the phonon mode of crystals, the low-frequency vibration mode of molecules, and the rotation mode of gases. Many amino acid and sugar crystals have THz resonance peaks for the hydrogen bonds between molecules in the crystal. The advantage offered by THz spectroscopy is that it can provide information on the non-covalent bond networks from the hydrogen bonds of biomolecules, organic molecular crystals, protein molecules that have higher-order structures, double-helix DNA (deoxyribonucleic acid), and other such molecules that dissolve in aqueous solution. [1], [2].
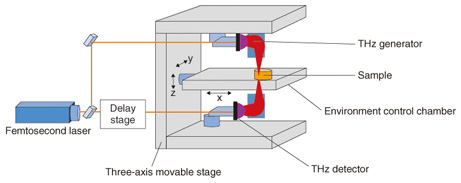
2. TCI analysis methodTerahertz chemical imaging (TCI), which visualizes the THz optical spectrum in two or three dimensions, differs from ordinary x-ray imaging in that it identifies molecules from the spectra of molecular networks. TCI can be used to investigate the non-covalent bond network of molecules as well as the molecular distribution, so it may provide a means of evaluating new medical diagnostics and pharmaceuticals. Systems for production process design, analysis, management, and final product quality assurance, generally referred to as process analytical technology (PAT), have already been introduced in the petroleum and chemical industries, and FDA (U.S. Food and Drug Administration) guidance calls for introduction of that technology to improve the quality of pharmaceuticals. The applicability of THz spectroscopy to PAT is also attracting attention. TCI analysis systems used in THz time-domain spectroscopy (THz-TDS) systems include those that use pulsed light, which can cover a wide frequency band, and those that use continuous light with frequency scanning [3]. A TCI analysis system that uses THz-TDS with an environment control chamber is illustrated in Fig. 3. An ultrashort-pulse laser beam is split into two beams, one of which is input to a THz wave generator and the other to a THz detector. The most typical THz wave generators and detectors are photoconductive antennas. The sample is illuminated with coherent sub-picosecond THz pulse waves, and the electric field strength of the passing waves is changed. Instead of measuring the strength of the light in the frequency domain, a delay stage is used to measure the electric field strength in the time domain. The THz pulse waves are measured repeatedly and converted to strength and phase values by Fourier transform. The waveform of the THz pulse wave is measured and recorded while scanning the stage, and the Fourier transform is used to convert the data to an image for each frequency. A feature of this system is that it can measure the images of the sample in an environmental control chamber. The temperature and pressure within the chamber can be controlled, so it is possible to change the non-covalent bond network of the molecules inside the crystal. As a result, the applications of TCI analysis now include investigating multi-component analysis, distinguishing active pharmaceutical ingredients (APIs) in diluents, and identifying differences in degree of hydration.
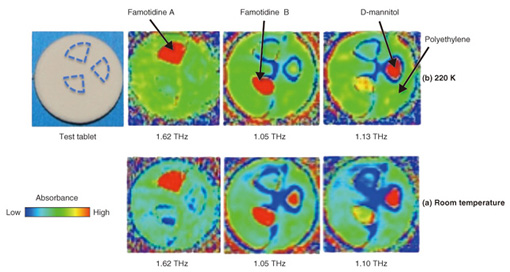
3. Identification of pharmaceutical crystals using TCI analysis systemAn example of using a TCI analysis system to distinguish between pharmaceutical crystal polymorphs and diluents is shown in Fig. 4 [4]. Crystal polymorphs consist of the same molecules but differ in how the molecules are linked. They thus can have large differences in solubility and other chemical properties that affect drug efficacy. Also, diluents such as cellulose and/or sugars, which themselves have no drug efficacy, are used when pressing pharmaceutical crystals into pills or tablets. Those diluents also often have high absorbance in the THz band, making identification difficult. Famotidine, a component of stomach medicines, has two crystalline forms, type A, which has low solubility, and type B, which has high solubility. Only the highly soluble famotidine B has drug efficacy. These two crystal polymorphs exhibit entirely different THz spectra and are therefore easily distinguished, but it is often difficult to distinguish the drug from the diluent. In this example of a test tablet, the peaks for D-mannitol and famotidine B are very close and cannot be distinguished in the image acquired at room temperature (Fig. 4(a)). However, if a low temperature of 220 K is maintained within the environmental control chamber, we can see clear separation of famotidine A, famotidine B, and D-mannitol as shown in Fig. 4(b). This is due to the differences in structural changes such as the distance between molecules in the crystals at different temperatures.
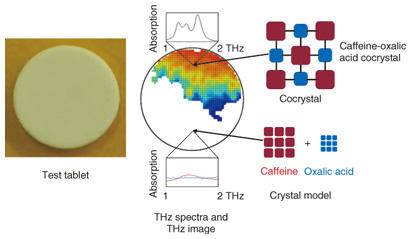
4. Analysis of crystal distributionAn example analysis of pharmaceutical cocrystals is shown in Fig. 5 [5]. An advantage of cocrystallization is the discovery of functions and properties that cannot be obtained with single-compound crystals. In particular, because improved solubility can lead to relaxed constraints on dose absorbability and selection of formulation, it is very important in exploratory research on drug development. TCI analysis has been attracting attention as an effective method for distinguishing high clarity cocrystals and the pharmaceutical molecules that constitute them, and additives as well, in the analysis of pharmaceutical cocrystals whose structure is stabilized by hydrogen bonds, and other interactions between and within molecules. In this example, we created a tablet model with an uneven distribution in which readily soluble oxalic acid is used as an additive for cocrystallization with insoluble caffeine, a drug that has a stimulating effect. We used a TCI system to analyze the cocrystal distribution in test tablets formed from a mixture of the active compound with polyethylene powder. Comparison of the THz spectra showed that the single compound caffeine or oxalic acid crystal exhibited almost no absorption, but the caffeine and oxalic acid cocrystal exhibited a particularly strong absorption peak at low temperature. We know from this result that it is possible to clearly distinguish a cocrystal and its constituent API and diluent. We also learned that the component distribution in a non-uniform tablet model can be visualized as a two-dimensional image by applying TCI analysis and using the characteristic absorbance peak data.
5. ConclusionWe described THz spectroscopy as a revolutionary technique for visualizing the molecular bonds in various forms of pharmaceutical crystals such as cocrystals and polymorphic forms. THz spectroscopy and TCI analysis have not come into widespread use because of the high equipment cost, but differences in molecular non-covalent bond networks can only be identified in the THz energy band. We can therefore expect further development of THz spectroscopy as a revolutionary technique for visualizing the molecular bonding that is closely related to pharmaceutical crystal solubility and systemic absorption rate but that has not previously been understood, and as a technique for carrying out early diagnosis of cancer and other such diseases. References
|
|||||||||||