|
|||||||||||||||||||
|
|
|||||||||||||||||||
|
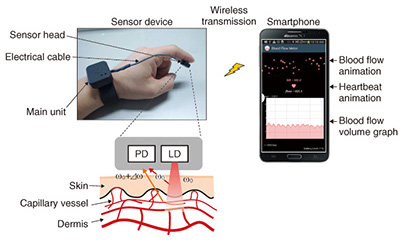
Feature Articles: R&D on Devices Using Life-assist Technologies Vol. 13, No. 1, pp. 17–22, Jan. 2015. https://doi.org/10.53829/ntr201501fa3 Blood Flow Observed with Smartphone— Ultracompact Wearable Blood Flow SensorAbstractThe flow of blood that circulates through the entire human body and supports life changes dynamically with our emotional and physical state and reveals a huge amount of information. If the flow of blood could be easily visualized in our daily lives, we could expect to see a range of useful applications in areas such as health, beauty, and sports. In this article, we introduce an ultracompact, wearable blood flow sensor that is linked to a smartphone, making it possible to view the flow of blood anywhere and at any time. Keywords: blood, smartphone, wearable 1. IntroductionThe adult human body is estimated to consist of several dozen trillion cells [1]. The oxygen and nutrients necessary for the life of these cells are supplied via the blood that circulates through the entire body. In addition, carbon dioxide and other waste products excreted from the cells are collected in the blood flow. The red blood cells, the major component of blood, account for a large proportion of the total cells in the human body, so we can say that our lives are supported by the flow of blood. Various health problems can occur if this vital flow is impeded. Heart disease and cerebrovascular disease, the second and third most common causes of death among Japanese people, are prime examples of this. Other bodily disorders that routinely worry many people, for example, chills, numbness, and body stiffness, are also thought to be caused mainly by obstructions in the flow of blood [2]. The importance of blood flow is also recognized in areas such as the beauty industry and sports medicine. Thus, the flow of blood is vital to human life, and if we could better comprehend it, it might lead to people rethinking their lifestyles and undertaking activities to improve blood circulation. However, it has not been possible for people to measure their blood flow routinely. This is because commercially available blood flow meters are large, impossible to carry around, and expensive, so they can only be used in hospitals and other such facilities. Consequently, we are applying technology acquired through NTT’s past communications-related research and development (R&D) to develop a portable, compact blood flow sensor [3]. We have extended this technology even further and linked this blood flow sensor to a smartphone, which will enable users to visualize the flow of blood in daily life over extended periods. 2. System configuration and operating principleThe configuration of our prototype blood flow sensor system is shown in Fig. 1. This system consists of a sensor device and a dedicated application for a smartphone. The sensor device consists of a sensor head and a main unit. A laser diode (LD) and a photo diode (PD)—a light-sensitive element—are installed in the sensor head. Infrared light from the laser shines on the skin, and light that is scattered back is detected by the PD. Light that strikes and is scattered by the red blood cells moving within the blood vessels generates a frequency shift that is proportional to the velocity of the moving red blood cells due to the Doppler effect of light, so it is possible to acquire information relating to the flow of blood by analyzing the frequency spectrum of the detected signal [4]. With this information, the smartphone provides visualizations such as a graph of the blood flow volume*1 or animations of the flow of blood and the beating heart.
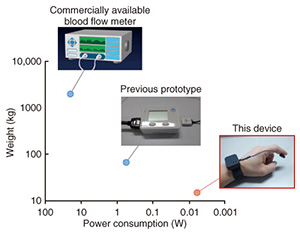
The weight and power consumption of this blood flow sensor are compared with those of a previous NTT prototype model and a commercially available blood flow meter in Fig. 2. The weight and power consumption of the previous NTT prototype model were far lower than those of the commercial blood flow meter. The weight was approximately one-thirtieth lower, and the power consumption was approximately one-fortieth lower. Our current prototype model reduces these values even further; the respective weight and power consumption are approximately one-quarter and one one-hundredth that of the previous NTT prototype. This enables a new form of use in that it can continuously monitor changes in the blood-flow during the day, while being easily carried around in daily life. There are two reasons we were able to achieve such huge reductions in weight and power consumption. The first reason is that some of the functions installed in the previous sensor device are now provided by the smartphone because the overall system design is based on a link with a smartphone. The second reason is that we modified the circuitry and signal processing so that the blood flow sensor would operate intermittently at high speed in order to achieve efficient operation. We explain these technologies in more detail in the following sections.
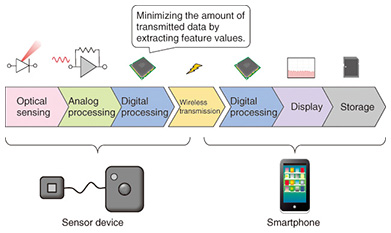
3. System design technology linked to smartphoneA blood flow sensor must have certain functions, for example, optical sensing, analog signal processing, digital signal processing, the capability to display the analysis results, and storage. In the past, sensor devices had to be equipped with all of these functions. However, this situation has changed greatly with the recent spread of smartphones. Smartphones come equipped with features such as a sophisticated processor, a high-definition display, a large-capacity memory, and communications functions, and therefore, we were able to install only the minimum necessary functions in the sensor device by actively applying other functions on the smartphone. A sensor that functions in integration with a smartphone in this way is called an appcessory (a portmanteau word combining application and accessory), and it has recently become a new trend in sensor development. To make the sensor device smaller and reduce its power consumption, it is important to optimize the sharing of functions between the sensor device and the smartphone. An image of the function-sharing of our prototype blood flow sensor is shown in Fig. 3. The sensor device implements the processing of raw data acquired by optical sensing—from amplification, filtering, and sampling, to the extraction of feature values by digital signal processing. It relies on a smartphone for other functions such as advanced digital signal processing and the display and storage of analysis results. Having the sensor device transmit the measured raw data (without processing it) to the smartphone would reduce the amount of data processing, but there is a trade-off in that the amount of data being transmitted would increase. Thus, we reduce the amount of communications data to less than one one-hundredth that of the case when the sensor device transmits the measured raw data to the smartphone, by transmitting feature values after extracting them from the raw data on the sensor device side, as described previously. This minimizes the power consumption of the sensor device.
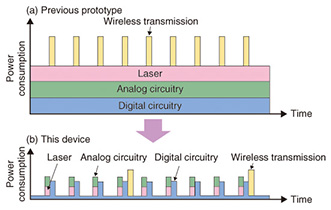
4. Reduced power consumption by intermittent operationThe operation and power consumption of the previous blood flow sensor and the blood flow sensor we have newly prototyped are shown in Figs. 4(a) and 4(b). In the previous blood flow sensor, the laser fires continuously during the measurement, the analog and digital circuitry also operates continuously, and the measurement results are transmitted frequently. In contrast, our new blood flow sensor implements efficient measurement operations, which substantially reduces power consumption. More specifically, the amplification and filtering of signals is done within a short time period by having the laser fire for only short periods intermittently and having the analog circuitry operate intermittently in tune with the laser. The digital circuitry also goes into sleep mode as soon as it has sampled the signal and extracted feature values in order to reduce power consumption.
For wireless communications, we adopted the Bluetooth*2 low energy (BLE) method, which can operate with low power consumption by using intermittent communications. BLE is a new communication method that is installed in Android*3 operating systems from version 4.3. This enables even lower power consumption than in previous versions of Bluetooth by transmitting small amounts of data, at a maximum of approximately 20 bytes per packet, only when necessary. With our newly prototyped blood flow sensor, we make the most of BLE communication capabilities and implement efficient communications by collecting the measured data in a buffer without transmitting it immediately, and then transmitting the data in a batch once a packet is full. In this way, we greatly reduce power consumption by running all the optical sensing, analog signal processing, digital signal processing, and wireless communication processing intermittently, making it possible to monitor the flow of blood over long periods of time with a small battery.
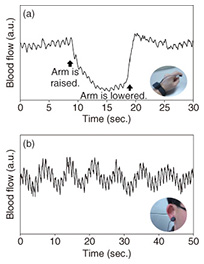
5. Examples of measuring blood flow volumeHere, we introduce examples of measuring blood flow volume using this blood flow sensor. The results of measuring blood flow volume when the blood flow sensor was attached to a fingertip and the arm was raised and then lowered are plotted in Fig. 5(a). It is clear that the blood flow volume at the fingertip drops when the arm is raised, due to the effect of gravity, but it returns when the arm is lowered. Thus, the flow of blood in a person changes dynamically with routine actions. The results of measuring blood flow volume at an earlobe are shown in Fig. 5(b). The blood flow volume at the earlobe has periodic fluctuations at approximately 1-s and 10-s intervals. The fluctuations at approximately 1-s intervals correspond to the heartbeat, and those in the approximately 10-s cycle represent the contraction motion of blood vessels, which is referred to as vasomotion. These fluctuations vary with certain conditions such as the physical or emotional state, and therefore, various applications of this phenomenon are being investigated [5].
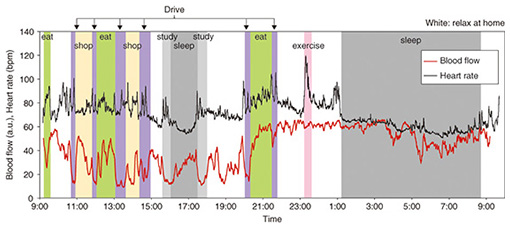
The results of measuring blood flow volume for 24 hours continuously during daily life are shown in Fig. 6. The figure also shows the simultaneously measured heart rate when the subject was wearing a hitoe wearable electrode inner shirt [6]. The effects on blood flow of daily actions such as driving, eating meals, and sleeping can be perceived. By taking such measurements, people who are concerned about bodily disorders caused by bad circulation can obtain information on improving their circulation in daily life. It used to be impossible to use a blood flow sensor to measure blood flow over long periods while going about our daily lives, but the features of the blood flow sensor we have newly prototyped, namely its small size and low power consumption, have made it possible to achieve this for the first time.
6. Future developmentThe flow of blood changes dynamically in response to our emotional and physical states, and it contains a vast amount of information. The fields of application of a wearable blood flow sensor that can measure the flow of blood anywhere and at any time cover a wide range including routine circulation monitoring, at-home medical care, beauty, and sports. We are actively promoting collaboration with businesses and universities that have strengths in these fields, and we are working on creating services that support people in living healthy and active lives. References
|
|||||||||||||||||||