|
|||||||||||
|
|
|||||||||||
|
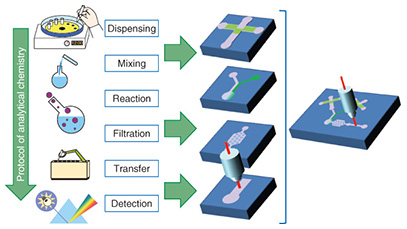
Feature Articles: R&D on Devices Using Life-assist Technologies Vol. 13, No. 1, pp. 23–28, Jan. 2015. https://doi.org/10.53829/ntr201501fa4 MicroTAS for BiosensorsAbstractMicro-total analysis systems (microTAS) are expected to be useful in daily healthcare applications in the near future because of their ability to measure biological information from molecules in the human body. This information is now assessed using sophisticated analytical systems at specialized institutes. MicroTAS is based on microfluidics, a multidisciplinary field that involves precise control of fluids at the sub-millimeter scale. A microfluidic device handles liquid just as an electronic device handles electricity. In this article, we introduce microTAS technology currently under development that makes measurement protocols as simple as possible through the use of microfluidics and enables end users to measure their health condition by themselves. Keywords: microfluidics, microTAS, biosensor 1. Introduction1.1 Overview of microTASAnalytical chemistry is the scientific field concerning measurement of chemical substances. Blood analysis carried out as part of a medical health checkup is based on analytical chemistry techniques. The primary discipline in analytical chemistry is carrying out chemical experimental protocols in a predefined way. These protocols include the handling of cuvettes, flasks, and pipettes. The basic idea of micro-total analysis systems (microTAS) is to replace those laboratory liquid management tasks with a liquid handling circuit device (microfluidics) and reduce an entire laboratory room down to one chip (Fig. 1) [1]. This is why microTAS is also frequently referred to as lab on a chip.
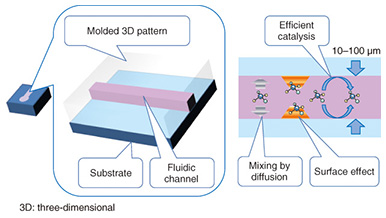
The typical liquid handling tasks in analytical chemistry include: dispensing, mixing, initiating a reaction, filtration, transfer, and measurement. Each task requires time and labor if the experiment is carried out using discrete tools and measurement instruments. The task will become more difficult as the sample volume is reduced. The use of microfluidic devices makes it possible to replace such liquid handling tasks with small channels, valves, and junctions. These liquid handling components are formed on a thin flat substrate, and the size is drastically reduced, so we can handle the sample liquid more easily and deal with small volumes automatically. 1.2 Microfluidic devicesMicrofluidic devices are fabricated using photolithography and microelectromechanical systems (MEMS) technology, the same technology as that used to integrate electronic circuit elements (registers, transistors, wiring) on a silicon wafer. Therefore, the microfluidic device and the internal volume can be fabricated with a small size. The microfluidic devices have a micrometer-scale trench structure in which liquid flows. Therefore, a very small volume of liquid can be handled and used for chemical reactions in these devices. These characteristics of microfluidic devices make them best suited for analyzing biological and medical samples that are difficult to collect in a large volume for sample analysis. Microfluidics technology has advanced to the level where elementary liquid handling functionality is achieved with a certain pattern or circuit of liquid flow. For example, mixing is achieved with a Y pattern, dispensing with a cross pattern, and filtration with a pillar array. These patterns are also constructed in a trench structure. These functional units are pieces of patterns in a plane structure, so we can combine and integrate the pieces as in a jigsaw puzzle to construct a chemical reaction device that carries out a specific and total chemical reaction, synthesis, and analysis instead of having to manually carry out these tasks with separate tools. For example, polymerase chain reaction (PCR) is a common process in genetic biochemistry that amplifies the specific sequence of deoxyribonucleic acid (DNA) concentration, and it is necessary in DNA analysis. A PCR protocol consisting of multiple complex chemical reactions was carried out automatically using microfluidic devices [2]. Because microfluidic devices are fabricated using MEMS mass production techniques, they can be made in arbitrary patterns and in large quantities with high dimensional precision. A transparent material is used for the liquid circuit structure (Fig. 2) to enable the liquid inside the microfluidic device to be observed in order to measure and check the liquid flow. Mold casting is a common method to make these structures, as it enables repeated fabrication of the devices.
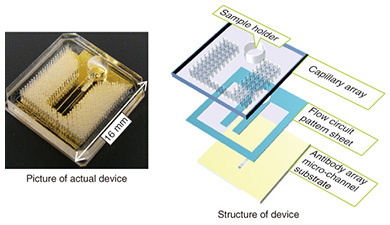
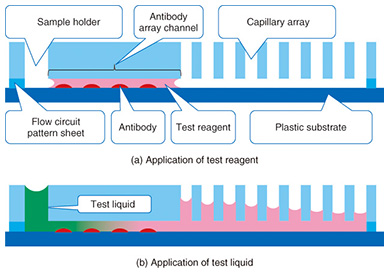
Typically, the trench in a microfluidic device has a cross-sectional length of 10–1000 μm. The shorter the length is, the smaller the internal volume is. This is advantageous for small volume reactions. In general, the area is proportional to the square of the length, but the volume is proportional to the cubic square of the length. Therefore, the ratio of volume to surface area of the internal microfluidics increases as the length gets shorter. This makes the effect of the internal wall of microfluidic devices very strong compared to macro-scale liquid handling tools. This is the same phenomenon as that of a straw placed in a glass of liquid; the narrower the straw is, the higher it can draw up the meniscus (the curve in the surface) of the liquid due to capillary force. With microfluidic devices, the smaller they are, the greater the capillary force governing the liquid behavior. Chemical reactions are usually facilitated by mixing, and mixing is a fundamental procedure in chemical analysis. In a microfluidic device, mixing is controlled by flow and diffusion. The flow in most microfluidic devices is very stable because liquid is held between walls that are spaced closely together, and the mixing is controlled by the fluidic pattern. When the internal wall is modified with a catalyst, the reaction efficiency is enhanced because the reactant has only a short distance to travel to access the catalyst [1]. 2. Biomolecule sensingCurrent healthcare diagnosis involves collecting biological samples (e.g., blood, urine) and analyzing the components to determine the patient’s state of health. In most local hospitals, the collected samples are analyzed using specialized massive-scale measurement instruments installed in a specialized medical test center that is usually located away from the hospitals. These instruments measure a large number of samples with high throughput; therefore the test cost becomes low enough to be covered by medical insurance. However, the patient or examinee cannot find out the results on the day of sampling and also cannot be tested more frequently than once a day to assess sudden changes. However, frequent measurement of concentrations of specific blood components is considered to be effective for controlling or reducing the risk of lifestyle related adult illnesses such as diabetes and stroke. For these purposes, a way for examinees to test themselves at home instead of going to a hospital is desired. This would also enable examinees to monitor changes to their state of health that may occur within a few days. This type of self-test should be easy to do and low in cost, and microTAS has the potential to help achieve this. Conventional microTAS chips [1] have been successfully used in liquid handling tasks. However, the pumps that enable the liquid to flow through the circuits and the detectors used for the reaction monitoring are still too large to realize personal measurement capabilities. To overcome this problem, we developed a low cost microfluidic device that is integrated with an autonomous liquid flow mechanism and a small-sized reaction detector; we describe it in the next section. 3. On-chip sample liquid flow systemWe developed an on-chip sample liquid flow system using capillary force and surface tension [3]. This device comprises a plastic substrate with antibody array, a capillary array formed in a plastic plate, and a plastic sheet with a flow circuit pattern (Fig. 3). These three parts are simply bonded by an adhesive agent on both sides of the sheet. The liquid circuits connect a sample holder, an antibody array micro-channel substrate, and a capillary array to each other. The internal surface of the fluidic device is hydrophilic. The device works as follows. An aqueous test reagent is introduced in the sample holder. Capillary force causes the test reagent to flow into the antibody array channel as in the principle of the narrow straw. The flow stops when the channel is filled if the volume of the reagent is set equal to the internal volume of the channel. In this stage, the capillary force of the head and tail of the liquid plug (i.e., a volume of liquid in air) is balanced (Fig. 4(a)). In this way, this device can control the liquid flow in the channel by itself.
When the test liquid is added to the sample holder, the capillary force of the tail of the liquid becomes lower compared to the head. Then the head moves to the capillary array. The test reagent and the test liquid that follows it flow under the many capillaries. The liquid is pumped up by the capillaries, and the test liquid flows continuously through the antibody array channel (Fig. 4(b)). This continuous flow enables the antibodies and molecules in the test liquid to react efficiently. The test sample, which may be a biologically hazardous substance, flows into the capillaries and stays in the device.
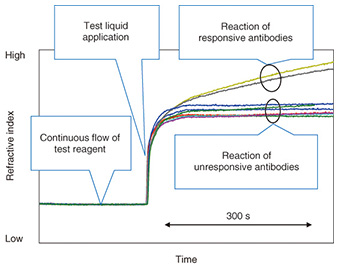
This device can be used with a small multi-point SPR (surface plasmon resonance) instrument* [4] that was developed by NTT. This instrument can detect subtle refractive index changes of the entire length of the channel at the same time. The refractive index changes are generally caused by the reaction of a molecularly thin film of immobilized antibodies to antigens in the channel. The instrument is sensitive enough to detect changes at the monomolecular-layer level. Therefore, antibody reactions in the antibody array channel can be easily detected. The developed device had nine antibodies of different specificities in the channel. After the test reagent was introduced, the refractive index recorded by SPR showed a flat response because no reaction occurred in the channel, as shown in Fig. 5. Then, after the addition of the test liquid, the refractive index increased because of the refractive index difference between the test reagent and the test liquid. This is an indication of the arrival of a sample. At this stage, the antigens in the test liquid started to react with antibodies in the channel. The reacted antibodies (yellow and black curves) showed a further increase in the refractive index. This is because the complex formation by a specific binding of antigen and antibody causes a high density region (high refractive index) in the channel. In contrast, the other seven curves showed a constant response, which is a result of no binding occurring between antibodies and molecules in the sample. In this way we can measure the pattern of response from nine antibodies in a simple operation. Because each antibody detects its specific biologically relevant molecule, we can evaluate the molecular components of the sample from the response pattern of antibodies. By changing the combination of antibodies, we can use this device for specific applications.
Moreover, because the raw data of the SPR instrument consist of a sequence of image data, the device can also detect sample introduction and flow conditions (e.g., whether the liquid is stuck, is leaking, or there has been a liquid introduction error) automatically through the use of sophisticated image processing technology. This automatic recognition helps end users to carry out correct measurements. We developed these technical elements using a simple sensor system.
4. Future perspectivesWe developed our on-chip sample liquid flow system with the aim of using it in on-site measurement in the dairy industry as a first step, for example, to identify the pathogens in milk samples on dairy farms. There has long been a demand in this field for a means of instant chemical analysis. In the next step, we are developing technologies enabling personal chemical analysis of biological samples that can be carried out as easily as making a phone call using microTAS. Data analysis of frequent measurements and a large number of examinees will make it possible to detect abnormal health conditions before they require costly treatment. We expect that the developed technology will be useful in providing new services to achieve improved health outcomes at a lower cost, and will expand the choice of treatments for each person. References
|
|||||||||||