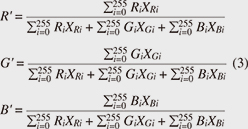|
|
|
|
|
|
Regular Articles Vol. 15, No. 11, pp. 40–45, Nov. 2017. https://doi.org/10.53829/ntr201711ra1 Optimization of Harvest Time in Microalgae Cultivation Using an Image Processing Algorithm for Color RestorationAbstractAlgae have been attracting attention as a next-generation alternative energy source to fossil fuels, but the high cost of cultivation remains an issue. This article introduces technology that reduces the cultivation cost through the use of an image-processing algorithm for color restoration to quickly determine the optimal time to harvest algae. Keywords: image processing algorithm, microalgae, color restoration 1. IntroductionGlobal warming has become a serious environmental issue. The application of photosynthesis using microalgae has come under focus as way to reduce the amount of carbon dioxide in the atmosphere, which is deemed to be the cause of the greenhouse effect. Microalgae absorb carbon dioxide, convert it to oil, and store it internally. This oil features high added value with applications to biofuels, moisturizing oils, and health food (Fig. 1).
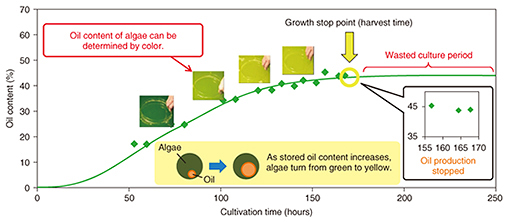
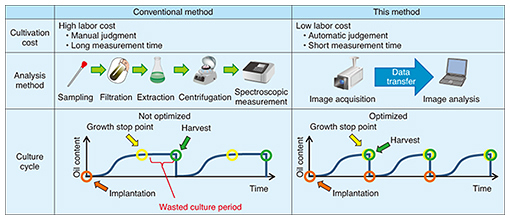
DENSO CORPORATION has begun cultivating a microalga called Pseudochoricystis ellipsoidea. Compared with terrestrial plants, microalgae have high growth potential and fast oil production on a par with light oils. These features make microalgae a good candidate for a next-generation, alternative energy source to fossil fuels. However, the current cultivation process is costly in comparison to the existing oil-producing process. In response to this issue, DENSO is conducting tests at a large-scale cultivation facility with the aim of reducing costs. 2. Key points in reducing cultivation costsAlgae have the property of stopping oil production after a certain growth period has been exceeded. Consequently, allowing cultivation to continue past this growth stop point results in unnecessary cultivation costs (Fig. 2). Harvesting at the growth stop point would therefore make for optimal cultivation.
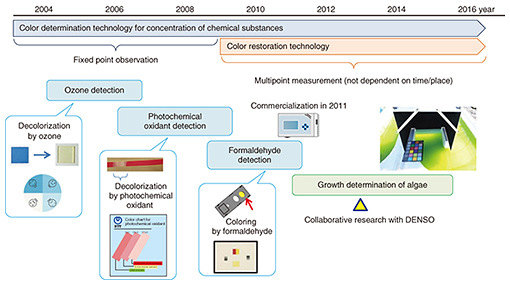
We point out here that the total amount and type of oil stored by microalgae depend largely on the environment. As a result, a difference in the growing environment even when cultivating the same type of algae will cause the weight percentage of stored oil to differ, which means that this value cannot be used as a simple index for assessing the cultivation time and determining the growth stop point. However, the color of the algae cultivation solution differs according to growth. In the early stage of cultivation, the number of cells is increasing, and the color green originating in chlorophyll, a photosynthesis pigment, is becoming darker. However, once the storage of oil begins and the constituent weight of that oil in the algae increases, the color of the algae itself begins to change from green to yellow. Accordingly, if the stop point in this color change can be accurately identified, it should be possible to determine the time at which oil production stops. 3. Development of color determination technology at NTTNTT has been developing color determination technology in relation to the concentration of chemical substances for some time and has applied this technology to environmental monitoring of ozone and other photochemical oxidants (Fig. 3). Furthermore, while studying methods for evaluating the growth of algae as a new application of this technology, we entered into a joint research project with DENSO.
The existing growth evaluation method involves sampling the algae cultivation solution and analyzing the components of the algae (Table 1). The entire process is done manually, which results in high labor costs and a time-consuming process that takes several days to complete from sampling to evaluation. The method introduced here, however, involves evaluating color using the RGB (red, green, and blue color model) information contained in an image of the algae. It enables simultaneous multipoint monitoring by capturing the algae over a broad area. In addition, connecting this method to a network enables remote operation and automatic measurements to be performed. Moreover, because image analysis can be performed in a short time with this technology, the time from determining the growth stop point to harvesting the algae can be shortened, substantially reducing the wasted cultivation period.
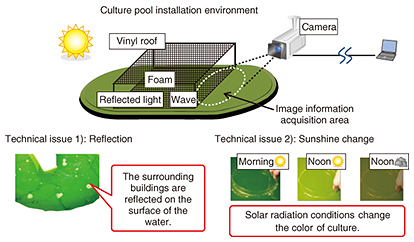
4. Technical issues in remote image analysisAlgae cultivation pools are installed outdoors, and therefore, reflections unrelated to components in the cultivation solution caused by foam on the solution surface and pool surroundings will affect the captured image. Additionally, because sunshine conditions can change from hour to hour, camera-shooting conditions cannot be fixed. These technical issues that prevent the correct determination of colors from the source image have an effect on remote image analysis (Fig. 4).
In response to the first issue, we suppressed reflections by installing a box coated with anti-reflective paint on the upper surface of the cultivation pool and shooting the surface of the cultivation solution from only the inner side of the box. We addressed the second issue by estimating the effects of sunshine at the time of image capture from RGB information and performing brightness correction and color-temperature correction against the image of the cultivation solution. The flow of these correction procedures is given below. 4.1 Step 1: Correction for sunshine brightnessSince colors cannot be accurately determined if the brightness of image information changes, we standardized the brightness value using the following procedure. First, we obtained the R, G, and B gradation (0–255) for each of the pixels in the measurement area (Eq. 1).
Next, we created a histogram of frequency and gradation for the pixels in the measurement area and calculated the product of gradation and gradation frequency X for each color component (Eq. 2).
Now, by applying Eq. 2 to all pixels and taking the sum total, we calculated the brightness of the entire image, and by standardizing the brightness of each R, G, and B measurement area against the brightness of the entire image, we standardized the brightness of each R, G, and B component (Eq. 3).
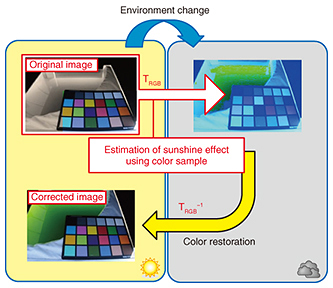
4.2 Step 2: Correction for sunshine color temperatureTo evaluate the effect of a change in sunshine color temperature on the RGB information in the algae and to correct for this effect, we installed a color sample board (Fig. 5).
We then obtained the RGB information of the image of this color sample board beforehand (Eq. 4) as a reference for shooting conditions; here, Rn denotes the R information of the nth color sample.
Next, by obtaining the RGB information of the color sample board captured under the shooting conditions to be targeted for correction, we were able to denote the change in color with respect to the reference RGB information using 3×3 matrix TRGB in Eq. 5, where R'n denotes the R information of the nth color sample to be corrected.
Then, by applying the inverse matrix of this color-change matrix TRGB to the RGB information of the cultivation solution targeted for correction (Eq. 6), we performed color restoration with respect to reference sunshine conditions. Here, R'algae is R information of the cultivation solution before correction, and R''algae is R information of the cultivation solution after correction.
4.3 Step 3: Determination of harvest timeThe color of the cultivation solution changes from green to yellow as the oil content percentage of the algae increases. In particular, we found that this increase in oil has a strong relationship with the percentage of R in the color of the solution. We therefore defined the percentage of R in the standardized RGB values using Eq. 7 and took this value of r to be an index for determining the harvest time.
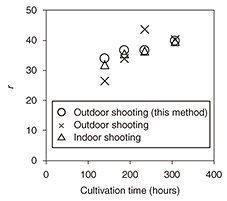
5. Experimental results and discussionWe collected some of the cultivation solution in an outdoor cultivation pool to compare the results of correcting for lighting conditions using the proposed method. The results shown in Fig. 6 compare RGB values (Δ) under fixed lighting (indoors), RGB values (×) under sunshine (outdoors), and RGB values (O) of an image captured under sunshine and corrected using the proposed method.
An examination of these results indicates that the r value under sunshine and that under fixed lighting show the most divergence, but that the r value after correction using the proposed method is nearly the same as that under indoor shooting conditions. These results demonstrate that the proposed method can restore an image captured outdoors to practically the same image captured in a disturbance-free indoor environment. 6. ConclusionIn this article, we introduced a method for determining in a short time the optimal harvest time for algae using a color restoration algorithm and obtained results that contribute to a reduction in cultivation costs. This method can be applied to any phenomenon for which a change in color occurs due to a change in constituent components. Going forward, we plan to expand the application domain of this method. |