|
|||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Regular Articles Vol. 20, No. 5, pp. 37–44, May 2022. https://doi.org/10.53829/ntr202205ra1 The Challenge to Develop an Artificial Photosynthesis Device that Fixes CO2 Using the SunAbstractInterest in reducing greenhouse gases, especially carbon dioxide (CO2), as a measure against climate change is increasing not only in countries but also in companies around the world. The NTT Group has formulated a new vision for zero environmental impact and declared its intention to achieve carbon neutrality by 2040. We expect artificial photosynthesis that converts CO2 and water (H2O) into hydrocarbons and molecular oxygen (O2) using solar energy to be a technology that contributes to CO2 reduction. To introduce this technology to the market, it is necessary to improve its efficiency and durability. We propose a gas-phase CO2 reduction reaction system to improve solar-to-hydrocarbon conversion efficiency ηSTC and conducting basic research on electrodes that make up this system. We studied a nickel oxide/indium gallium nitride (NiO/InGaN) photoanode to achieve both high efficiency and long lifetime. We also studied a copper (Cu)-fiber cathode to improve efficiency. In our system using these electrodes, formic acid (HCOOH) was produced in 140 hours of continuous light irradiation, resulting in an ηSTC of 0.16%. Keywords: CO2 reduction, renewable energy, GaN 1. IntroductionClimate-change awareness is growing worldwide, even with the COVID-19 pandemic. In 2021, the World Economic Forum reported that both the likelihood and expected impact of environmental risks, for example, climate-action failure, extreme weather, and biodiversity loss, are greater than those of economic, geopolitical, societal, or technological risks [1]. Greenhouse gas reduction in particular is being promoted as a measure against climate change. Japan announced at COP26* that in 2030 it will have reduced its greenhouse gas emissions by 46% from those in 2013 and by 2050 will have become a carbon-neutral society. Therefore, in Japan, renewable energies, such as solar power, wind power, geothermal power, small and medium-sized hydropower, and biomass conversion, are attracting increasing interest as promising and diverse energy sources. The NTT Group has formulated a new vision called “NTT Green Innovation Toward 2040” for achieving zero environmental impact through the combination of increasing the use of renewable energy and decreasing energy consumption with IOWN (Innovative Optical and Wireless Network) technologies. Our team is conducting basic research on artificial photosynthesis to contribute to carbon dioxide (CO2) reduction. Artificial photosynthesis is a technology that converts CO2 and water (H2O) into hydrocarbons (formic acid (HCOOH), methane (CH4), alcohols, etc.) and molecular oxygen (O2) using solar energy. Currently, CO2 capture and storage is expected to reduce CO2, but it is a large-scale system and its installation location is limited, especially in Japan. Artificial photosynthesis, which has scalability and is driven by sunlight, is expected to be a new method for CO2 reduction. With the development of artificial photosynthesis technology, we envision that artificial photosynthesis systems can be attached to buildings, vehicles, etc. to fix CO2 from the atmosphere, resulting in a more sustainable society.
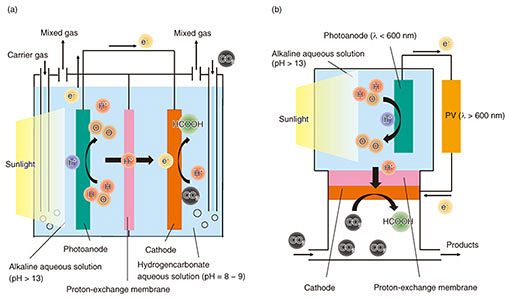
2. Artificial photosynthesis for CO2 reductionAn artificial photosynthesis system is mainly composed of a photoanode (negative electrode), cathode (positive electrode), and proton-exchange membrane (electrolyte), as shown in Fig. 1(a). When the photoanode, which is a semiconductor called a photocatalyst, is irradiated with light having an energy larger than the band-gap energy of the semiconductor, electron-hole pairs are generated in the semiconductor, resulting in an oxidation-reduction (redox) reaction. The principle of this system is based on the Honda-Fujishima effect [2]. When high-concentration CO2 gas is supplied to this system and light is applied to the photoanode, a water-oxidation reaction and CO2-reduction reactions can proceed as follows: Oxidation reaction: 2H2O + 4h+ → O2 + 4H+, Reduction reactions: CO2 + 2H+ + 2e− → HCOOH, CO2 + 2H+ + 2e− → CO + H2O, CO2 + 6H+ + 6e− → CH3OH + H2O, CO2 + 8H+ + 8e− → CH4 + 2H2O, 2CO2 + 12H+ + 12e− → C2H4 + 4H2O. The target performance for market introduction is 10% solar-to-hydrocarbon conversion efficiency ηSTC and 10-year durability in the 2030s. The ηSTC of CO2-reduction reactions is written as follows: ηSTC = {(nHCOOH × QHCOOH) + (nCO × QCO) + …} / (solar energy) × 100%, where n is the amount of product and Q is the heat of formation. Some research institutes have achieved an efficiency of 10% [3], but most teams have achieved percentages less than that. In addition, no research institute has reported that both efficiency and durability are compatible. Achieving both high efficiency and long lifetime of artificial photosynthesis systems is therefore an important issue in research and development. To improve the ηSTC, it is important to increase the Faradaic efficiency of the CO2-reduction reaction by increasing the CO2 supply to the reaction area for suppressing the side-reaction ratio [H+ + e− → H2]. The Faradaic efficiency is the ratio of the number of electrons consumed in the objective reaction to that generated in the photoanode. Even if many electrons are generated in the photoanode, if the CO2 supply is insufficient at the surface of the cathode, they will be consumed as the side reaction proceeds. In the conventional reaction system shown in Fig. 1(a), CO2 is dissolved in an aqueous solution and supplied to the reaction area on the surface of the cathode. The amount of CO2 supplied is limited by the solubility and diffusion coefficient of CO2 in the solution, and it is difficult to suppress the side reaction. We propose a gas-phase CO2-reduction reaction system to solve this issue, as shown in Fig. 1(b). It is easier to increase the concentration and diffusion coefficient of CO2 in the gas phase than it is to increase them in the dissolved phase. Thus, this system can increase the CO2 supply to the reaction area and improve the Faradaic efficiency of CO2-reduction reactions. In addition, a photovoltaic (PV) power generator that uses the light transmitted through the photoanode is connected in series to increase the photocurrent by applying a voltage corresponding to the overvoltage (Fig. 2). We are conducting basic research to improve the ηSTC and lifetime, focusing on the materials and structures of the photoanode and cathode.
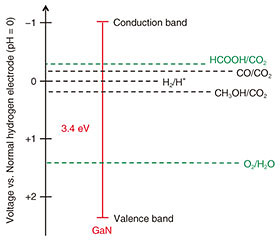
2.1 NiO/GaN-based photoanodeThe photoanode in an artificial photosynthesis system generates electrons for the proton and CO2-reduction reactions and generates holes for the water-oxidation reaction. The surface of the photoanode is also a water-oxidation reaction area. We focus on a gallium-nitride (GaN)-based photoanode [4]. The band-gap energy of GaN is 3.4 eV, and the top of the valence band is lower than the oxidation potential of water, and the bottom of the conduction band is higher than the reduction potential of protons and CO2 (Fig. 3). An aluminum gallium nitride (AlGaN)/silicon (Si)-doped GaN (n-GaN) heterostructure and indium gallium nitride (InGaN)/n-GaN heterostructure can improve the ηSTC because of enhanced electron-hole separation due to the large polarization field in AlGaN and enhanced electron-hole generation due to the wide absorbable wavelength range in InGaN. Thus a GaN-based photoanode is expected to generate O2 and hydrocarbons. However, there are still issues with improving the ηSTC and durability toward the target performance.
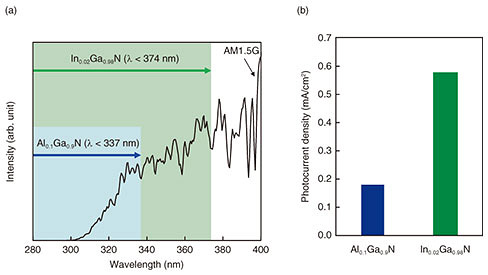
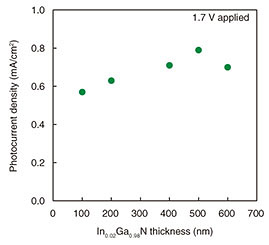
To improve the ηSTC, we investigated how to increase the light absorption of the photoanode material. Indium gallium nitride is a mixed crystal of GaN and indium nitride (InN), and its band-gap energy can be adjusted by changing its In composition. Therefore, as shown in Fig. 4(a), InGaN can use a wider wavelength range for the redox reaction than AlGaN. We prepared an In0.02Ga0.98N photoanode by growing an In0.02Ga0.98N/n-GaN heterostructure on a sapphire substrate. We measured the photocurrent in the hydrogen (H2)-production system, which is the simplest reduction-reaction system using platinum (Pt) as a cathode, under pseudo-sunlight irradiation. The photocurrent density, which is photocurrent per unit light-irradiation area, measured using a 100-nm-thick In0.02Ga0.98N photoanode, was higher than that measured using a 100-nm-thick Al0.1Ga0.9N photoanode (Fig. 4(b)) [5]. This suggests that expanding the wavelength range increases the photocurrent and that narrowing the band-gap energy of the photoanode material can further increase the photocurrent. We also studied the photoanode structure to further increase light absorption. The light absorption of an In0.02Ga0.98N layer increases with increasing layer thickness because of decreasing light transmittance. Figure 5 shows the dependence of photocurrent density on In0.02Ga0.98N-thickness in an H2-production system to which 1.7 V was applied under pseudo-sunlight irradiation [6]. The photocurrent density increased with increasing In0.02Ga0.98N thickness, and the maximum density was at 500 nm. Increasing the In0.02Ga0.98N thickness increases the photocurrent density by increasing the amount of electron-hole pairs. It also promotes electron-hole pair recombination caused by the lattice defects in In0.02Ga0.98N, thus reducing the photocurrent density. It is thought that the photocurrent density maximized at 500 nm due to the balance between increasing and decreasing the amount of electron-hole pairs.
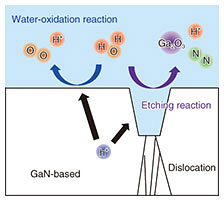
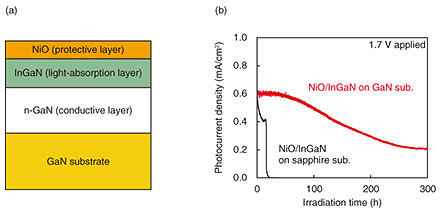
To improve durability, we investigated how to prevent the etching reaction [2GaN + 3H2O + 6h+ → Ga2O3 + 6H+ + N2]. The etching reaction is driven by holes generated in GaN-based photoanodes and continues at the interface between the GaN and aqueous solution, as shown in Fig. 6. As this reaction progresses, electron-hole pair recombination is promoted by the decrease in the crystallinity of GaN, thus, decreasing the photocurrent. We therefore formed a protective layer on the GaN-based photoanode to eliminate the interface between the GaN and aqueous solution [7, 8]. Nickel oxide (NiO), which is known to transport holes, was used for the protective layer. The thin film of NiO, about 2 nm thick, was formed to sufficiently transmit light. The reaction using NiO/InGaN photoanodes continued for about 20 hours, even though that using bare-InGaN photoanodes had been inactivate for several hours. We also studied the photoanode structure to find how to further suppress the etching reaction [9, 10]. The etching reaction continues with dislocations near the InGaN surface as the starting point. Thus, we prepared a photoanode with reduced dislocations by growing an In0.02Ga0.98N/n-GaN heterostructure on a GaN substrate instead of a sapphire substrate, as shown in Fig. 7(a). The dislocation density of the In0.02Ga0.98N/n-GaN heterostructure estimated from the full width at half maximum measured with an X-ray rocking curve decreased by an order of magnitude, and the surface roughness also improved. The stability of the photocurrent improved significantly, and the reaction continued for over 100 hours, as shown in Fig. 7(b).
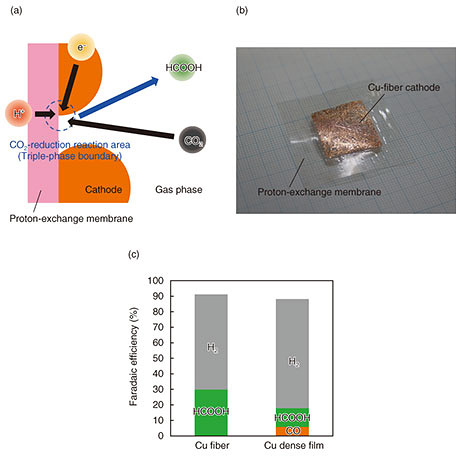
2.2 Metal-fiber cathode on proton-exchange membraneThe cathode catalyzes the CO2-reduction reaction. Some metals have been reported to catalyze CO2-reduction reactions, and it is known that the type of hydrocarbons produced differs depending on the type of metal. In our gas-phase CO2-reduction system, the CO2-reduction reaction continues in a triple-phase-boundary, as shown in Fig. 8(a) [11]. Therefore, it is also necessary to control the CO2 diffusivity in the cathode to increase the amount of CO2 supplied to the triple-phase boundary. When the cathode was formed on the proton-exchange membrane by using the plating method, the H2-generation reaction was dominant and ηSTC was low. This was because the CO2 supply to the triple-phase boundary was insufficient due to the dense electrode. To improve the ηSTC, we studied the metal structure to increase CO2 diffusivity in the cathode. Gas diffusivity generally depends on the porosity in the electrode. Thus, we focused on a metal-fiber structure with a large void easily controlled by changing the fiber diameter. The metallic fiber is formed on the proton-exchange membrane with a hot press under a condition that maintains the fiber structure, as shown in Fig. 8(b) [12]. Figure 8(c) shows the Faradaic efficiency of each product obtained using the copper (Cu) fibers and Cu dense film as the cathode in our gas-phase CO2-reduction reaction system. In this system, HCOOH was mainly generated in the CO2-reduction reaction and H2 was also generated as a by-product. The Faradaic efficiency of HCOOH obtained using the Cu-fiber cathode improved compared with that of HCOOH and CO obtained using a Cu-dense-film cathode.
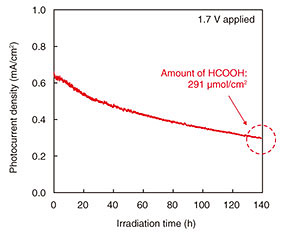
2.3 Gas phase CO2-reduction reaction using NiO/InGaN photoanode and Cu-fiber cathodeWe conducted photoelectrochemical measurement using a NiO/In0.02Ga0.98N photoanode grown on a GaN substrate and a Cu-fiber cathode on a proton-exchange membrane in our gas-phase CO2-reduction reaction system. As shown in Fig. 9, the photocurrent density with 1.7 V applied decreased to about half the initial value after 140 hours of light irradiation. The reduction products were HCOOH and H2. The amount of HCOOH produced 1 and 140 hours after beginning light irradiation were respectively 3.79 and 291 µmol. The ηSTC and Faradaic efficiency of HCOOH 1 hour after beginning irradiation were respectively 0.25 and 28%, and the average ηSTC and Faradaic efficiency of HCOOH 140 hours after beginning irradiation were respectively 0.16 and 24%. The amount of fixed carbon per unit light-irradiation area calculated using the chemical reaction formula for HCOOH production was 3.5 mg/cm2.
3. ConclusionWe proposed the gas-phase CO2 reduction system to improve the solar-to-hydrocarbon conversion efficiency ηSTC of artificial photosynthesis. Focusing on the materials and structures of the photoanode and cathode in this system, we are conducting basic research to improve its characteristics. We studied an InGaN photoanode and its thickness to improve the ηSTC by increasing the light absorption. We also studied a NiO protective layer and lower dislocations of InGaN to improve durability by suppressing the etching reaction of InGaN. We studied a metal-fiber cathode for improving the ηSTC by increasing CO2 diffusivity in the cathode. Using our gas-phase CO2-reduction reaction system with a NiO/InGaN photoanode grown on GaN substrate and Cu-fiber cathode, HCOOH was produced in 140 hours of continuous light irradiation, and the ηSTC was 0.16%. For future work, we will study the combining PV as the visible-light response elements with our system for improving the ηSTC and the system design for improving durability. We aim to achieve CO2-fixation performance better than that of a plant. References
|
|||||||||||||||||||||||||||