|
|||||||||||||
|
|
|||||||||||||
|
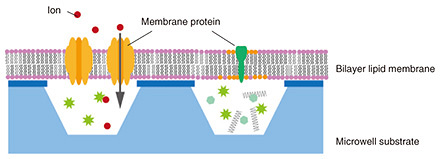
Feature Articles: Recent Updates on Bio-soft Materials Research Vol. 22, No. 5, pp. 39–45, May 2024. https://doi.org/10.53829/ntr202405fa4 Functional Evaluation of Bilayer Lipid for the Development of Artificial Cell-membrane StructuresAbstractLipids and membrane proteins are related in many reactions such as intracellular vesicular transport and intercellular communication. We are studying artificial cell membranes as a bottom-up approach to understand cellular reactions at the molecular level. Various elemental technologies are required to introduce membrane proteins into bilayer lipid membranes, which are the basic structure of cell membranes, and evaluate them. In this article, we introduce our efforts in developing an artificial cell membrane. Keywords: bilayer lipid membrane, membrane protein, cell 1. Reconstruction of bilayer lipid membraneBiological cells are covered with a lipid membrane that separates the inside and outside of the cell. The cell membrane contains a variety of membrane proteins, which adhere to or penetrate lipid membranes and account for almost 50% of the cell-membrane weight. Membrane proteins include receptor proteins that receive signal molecules such as neurotransmitters and transporters that transport ions using energy. Ion-channel-type receptor proteins are used for signal transduction by inducing a conformational change in the receptor due to ligand binding, which opens an ion channel, allowing ions to enter the cell and induce a change in electrical potential. Information transmission in the body is essential for vital activities, and abnormalities in information transmission can cause a variety of diseases. Thus, membrane proteins and lipid membranes, which are the basis for maintaining their functions, are closely related to biological functions. Understanding their dynamics is important not only in the life sciences but also in medicine and drug discovery. One method of evaluating lipids and proteins involves using bilayer-lipid-membrane reconstruction systems. Such systems assemble target lipids and membrane proteins under controlled solution and temperature conditions to reproduce phenomena on specific cell membranes and evaluate them at the molecular level. The lipid molecules that compose the membrane have a hydrophilic head and hydrophobic hydrocarbon chains and are insoluble in water. In solution, lipid molecules form a lipid bilayer structure, in which a monolayer of lipid molecules overlaps two monolayers to confine hydrophobic groups inside by self-accumulation. We previously proposed a simple cell model for functional evaluation of lipids and proteins by combining bilayer lipid membranes and well-shaped substrates fabricated using nano- and micro-scale processing techniques (Fig. 1) [1]. This artificial cell membrane consists of a freestanding bilayer lipid membrane (freestanding membrane) area at the well and a support bilayer lipid membrane (supported membrane) area on the substrate, and membrane proteins are introduced into the freestanding membrane area. The inside and outside of the well are separated by the freestanding membrane, which mimics the inside and outside of a cell. The well structure is approximately the same size as a cell. The bilayer lipid membrane covering the well is supported by a solid substrate, but the lipid bilayer and substrate are not physically adsorbed and exist through a water layer a few nanometers thick. It is also known that each lipid molecule that constitutes the bilayer lipid membrane is constantly moving, and the state of the bilayer lipid membrane can be evaluated using this lipid movement as an indicator. We discuss our evaluation of lipid-phase separation and changes in the bilayer-lipid-membrane shape using artificial cell-membrane substrates, the introduction of membrane proteins into bilayer lipid membranes, and the steps required to evaluate the ions that pass through the introduced membrane proteins.
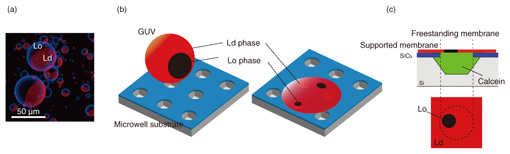
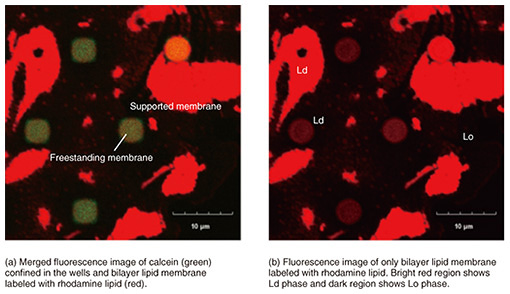
2. Lipid-phase separation membraneBiological cell membranes are composed of a variety of lipid molecules, which are roughly categorized into saturated and unsaturated lipids. These lipid molecules do not mix uniformly in the membrane, but saturated lipids are thought to form local domains with cholesterol present in the cell membrane. These lipid domains, called rafts, are in a different phase state than the surrounding membrane, and proteins involved in signal transduction are localized there. The roles and functions of lipid rafts need to be elucidated. However, since it is difficult to observe them directly in cell membranes, bilayer-lipid-membrane reconstruction systems have been used to understand the movement of lipid molecules as a physical phenomenon. A ternary mixture of saturated lipids, unsaturated lipids, and cholesterol produces a lipid-phase separation of a liquid ordered (Lo) phase/liquid disordered (Ld) phase. The Lo phase is less fluid and has a higher molecular density than the Ld liquid phase. We observed how such a phase-separation membrane behaves when formed on the well. To form phase-separated membranes on well structures, giant unilamellar vesicles (GUVs) were prepared from the ternary mixture of lipid solution. To distinguish each phase, the Lo and Ld phases were fluorescently labeled with laudan and with lipid conjugate with rhodamine, respectively. Figure 2(a) shows a fluorescent image of the prepared GUV. The blue areas indicate the Lo phase and red areas the Ld phase. When this GUV was ruptured on the well substrate, as shown in Fig. 2(b), a bilayer lipid membrane sealing the well was formed. To determine whether a lipid bilayer had formed, the fluorescent dye calcein was sealed inside the well (Fig. 2(c)). Figure 3 shows a fluorescent image of the phase-separation membrane on the well substrate. Red indicates an Ld phase, while the dark region indicates an Lo phase. The green fluorescence of calcein confirms whether the bilayer lipid membrane had successfully sealed the wells (Fig. 3(a)). The fluorescence image filtered for calcein-derived green fluorescence is shown in Fig. 3(b), and red fluorescence was observed in all the wells that were successfully sealed. This indicates that the Ld phase is preferentially present in a freestanding membrane, and the Lo phase is present only in the supported membrane [2]. Immediately after GUV rupturing, the Lo-phase areas were also observed in the freestanding membrane but decreased with time as they moved in the freestanding membrane. The Ld-phase area increased and the Lo-phase area disappeared from the freestanding membrane. This indicates that the lipids that form the low-fluidity Lo phase (saturated lipids and cholesterol) moved from the freestanding to the supported membrane, while the lipids that form the high-fluidity Ld phase (unsaturated lipids) moved into the freestanding membrane. Since the freestanding membrane is not in contact with the substrate, there is no substrate-lipid interaction as in the supported membranes, and the lipid molecules can move easily. The freestanding membrane is not supported, so it should be flexible in shape. These results indicate that even in a single continuous bilayer lipid membrane, each lipid has its own environment in which it is likely to exist. If we can control the movement of lipids by clarifying the conditions under which the Lo and Ld phases are likely to exist, we will be able to reproduce a wider variety of cell-membrane reactions and construct a cell-membrane model that is closer to living organisms.
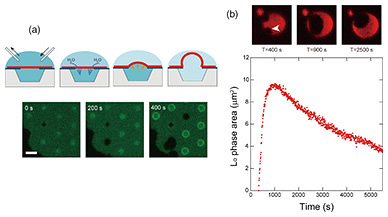
3. Shape changes in bilayer lipid membraneIn our artificial cell membrane, the Ld phase is preferentially present in the freestanding membrane. We observed how the phase separation changes when the shape of the freestanding membrane is changed. The inside and outside of the well are separated by a bilayer lipid membrane, which allows the solution to be exchanged during measurement. It is difficult for ions and large, uncharged polar molecules (amino acids and glucose) to permeate through the lipid bilayer, but small molecules, such as H2O and O2, permeate. As shown in the schematic in Fig. 4(a), when the solution outside the well is exchanged to cause an osmotic pressure difference between the inside and outside of the well, H2O flows into the inside of the well through the bilayer lipid membrane to cancel the osmotic pressure difference, and the solution volume inside the well increases [3]. Because of the fluidity of the bilayer lipid membrane, the lipid moved from the support membrane area and the freestanding membrane expanded like a balloon (Fig. 4(a), fluorescence image). When there was an osmotic pressure difference caused by the ternary mixture lipid bilayer of saturated lipid, unsaturated lipid, and cholesterol, the Lo-phase area, indicated with the arrow in Fig. 4(b), increased as the freestanding membrane expanded, although the Lo-phase area decreased in the isobaric state without osmotic pressure difference. This is because the lipids (saturated lipids and cholesterol) that form the Lo phase in the supported membrane surrounding the freestanding membrane moved into the freestanding membrane as the solution volume inside the well increased. After the freestanding membrane had finished expanding and most of the freestanding membrane was converted to the Lo phase, the Lo phase was successfully maintained in the freestanding membrane for more than one hour [4] (Fig. 4(b)). When the shape of the freestanding membrane was changed, a change in the movement of the lipids was observed. This method of physically controlling the movement of bilayer lipid membranes is important for studying the factors that affect their state.
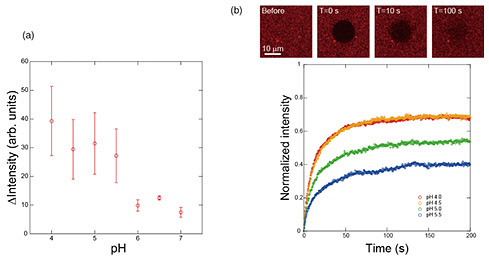
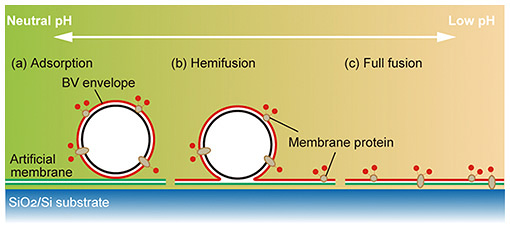
4. Membrane-protein introduction using membrane-fusion function of budding viruses from insect cellsIt is an important in reconstruction systems to efficiently introduce membrane proteins into bilayer lipid membranes. If membrane proteins can be placed in bilayer lipid membranes in a controlled number and orientation while maintaining their function, many cell-membrane reactions can be reproduced. Baculoviruses use insect cells as hosts. Since baculoviruses are covered by the insect cell membrane when they bud out of insect cells, the expressed membrane protein is added to the insect-cell-derived budding virus (BV). Insect-cell-derived BVs have the ability to fuse with cells to invade the next host. We focused on this function and applied it to the introduction of membrane proteins into bilayers lipid membranes [5]. We labeled the BV from insect cells with fluorescence, added it to the lipid bilayer formed on a silicon substrate, and examined changes in the fluorescence intensity of the labeled BV on the bilayer lipid membranes in solutions of different pH. Figure 5(a) shows the change in fluorescence intensity after one hour. The horizontal axis indicates the pH of the solution, and the fluorescence intensity of the dye labeled with the BV increases when the pH falls below 5.5, indicating an increase in the amount of BV reaching the bilayer lipid membranes. This indicates an increase in the amount of BV reaching the bilayer lipid membranes. A pH between 6.0 and 5.5 seems to be the threshold for activating the fusion ability of the BV. However, there is no significant difference in fluorescence intensity under pH 5.5. When bilayer lipid membranes fuse together, there are three stages of fusion: adherent, semi-fused, and fully fused. In some cases, the fusion proceeds no further than the adhered state. Since our goal is to introduce membrane proteins into bilayer lipid membranes, it is preferable that the membrane components derived from the BV are fully fused to them, but we cannot determine at what pH this is achieved from the results of fluorescence-intensity changes alone. Therefore, we evaluated the transfer of the components of the BV to the lipid bilayer by analyzing the lateral diffusion of lipids. Fluorescence recovery after photobleaching is a general method to evaluate the movement of lipids and components in a bilayer lipid membrane by analyzing the fluorescence-recovery curve obtained from the process of fading a fluorescent dye at a specific location by intense light and replacing the surrounding fluorescent dye with the faded dye by lateral diffusion. Figure 5(b) shows the fluorescence image of the movement of the quenched fluorescent dye by lateral diffusion. The fluorescence-recovery curve for each pH was obtained from the change in brightness of the fluorescence image, and the fluorescence-recovery rate increased at lower pH. This indicates that the amount of total fusion increases at lower pH due to the increase in the ratio of the membrane components of the BV that are transferred and diffused into the bilayer lipid membrane on the silicon substrate. The relationship between the pH of the solution and state of membrane fusion is shown in Fig. 6, which indicates that the ratio of membrane attachment is high around pH 5.5, and the ratio of total fusion increases as the pH decreases. In our artificial cell membrane, a solution pH under 4.5 is the optimum condition for membrane-protein incorporation. Under these solution conditions, we succeeded in introducing membrane proteins into the freestanding membrane area at the well.
5. Toward electrophysiological detection of ion-channel activity on deviceThe next step is to evaluate the function of the introduced membrane proteins. We measured ion flow through α-hemolysin, a membrane protein that forms pores in lipid bilayers and enables ion permeation, by using an artificial cell-membrane substrate. However, there is ion leakage from the external solution into the inside well, and this leakage must be reduced to detect signals of picoampere-level ion influx, as is the case with receptor membrane proteins. From theoretical and experimental investigations, it was found that the source of ion leakage is the inflow of ions from the interfacial water layer as small as 2 nm between the lipid bilayer and well substrate. We previously proposed a method of reducing ion leakage by surface modification of the substrate surface with bovine serum albumin to block the interface between the interfacial water layer and external solution, which is the path of ion leakage [6]. We have also found a simple and easy method of using dilute ionic liquid solutions as the aqueous solution. Ionic liquids are liquid salts composed only of ions, which are generally bulky organic ions. We succeeded in significantly reducing ion leakage through the interfacial water layer by using ionic liquids as electrolytes instead of ordinary inorganic salts and confining the bulky ions within the interfacial water layer. Using the lipid bilayer on the well substrate formed with these techniques, we successfully measured the ion-permeation function of α-hemolysin for more than a few hours and with high sensitivity. 6. Future developmentLipids and membrane proteins are not only related in intercellular communication but also in many reactions such as intracellular vesicular trafficking. We aim to develop a method of evaluating and controlling the movement of lipids and membrane proteins and reproducing the reactions that occur in cells to quantitatively evaluate the role of each biomolecule, which is difficult to visualize in cells. By controlling the movement of biomolecules, such as lipids and membrane proteins, with NTT’s substrate fabrication technology that can be adapted to biomaterials, we expect to develop a bilayer-lipid-membrane reconstruction system that enables the understanding of complex transmission pathways in the body through a bottom-up process. References
|
|||||||||||||