|
|||||||||||||
|
|
|||||||||||||
|
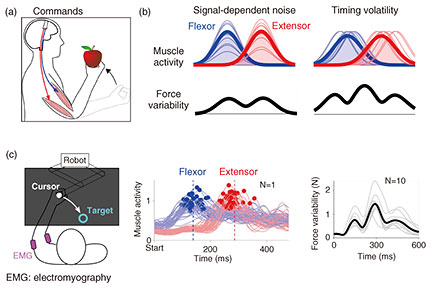
Feature Articles: Exploring the Nature of Humans and Information for Co-creating Human-centered Technologies with AI Vol. 22, No. 11, pp. 41–46, Nov. 2024. https://doi.org/10.53829/ntr202411fa5 The Crux of Human Movement VariabilityAbstractDespite intense training, even a seasoned baseball pitcher has difficulty throwing a ball to the same location repeatedly. I describe my research team’s finding that such movement variability comes from muscles that activate at imprecise times and introduce a new method that uses a smartphone to robustly quantify movement variability, which is tightly related to the arm muscles’ timing precision. This useful method reveals how movement variability, a measure of dexterity, changes with growth and age, and it quantifies the degree of handedness and footedness. Keywords: motor control, movement variability, timing 1. The importance of understanding how the brain moves the body as intendedHumans learn to move skillfully by activating the muscles on the basis of the sensory information the brain receives. Unlike robots, however, even the movements of skilled athletes are subject to variability, as movements cannot be repeated with perfect precision. This movement variability has long been the focus of attention in neuroscience. To understand the mechanism of information processing in the brain that enables us to move our hands and feet freely, it is necessary to investigate how the variability of movement changes with growth and training and consider the mechanism behind these changes. NTT has been conducting research to understand the brain information processing involved in sensory and motor generation to develop information and communication technology that can be used by people. I introduce my research team’s research into the nature of movement variability, which is deeply related to the ability of people to move as intended. 2. Previous researchPeople perform a variety of movements in their daily lives, such as throwing a ball or climbing stairs. In these movements, it is necessary to be able to move in the same way each time in accordance with the purpose of each movement. The mechanism of motor learning to achieve goal-directed movements has been studied extensively, and the information-processing mechanism of the brain has been clarified in previous research [1]. It is also known that movement variability decreases as motor learning progresses, but the mechanism that produces movements with less variability has not yet been elucidated. Humans move by activating the muscles spanning a joint. Since it is known from previous research that the muscle commands to generate muscle activity are, in principle, subject to variation, and since the variability of muscle activity increases with its magnitude, the prevailing idea was that the origin of movement variability comes from such signal-dependent noise [2]. According to this theory, the greater the muscle activity, the greater the muscle-force fluctuation. Imagine a scenario in which the elbow is flexed to bring an apple to the mouth. The elbow accelerates by increasing the activity of the muscle working in the direction of movement (the flexor muscle) then decelerates by activating the antagonist muscle. The force exerted by the flexor and extensor muscles will peak at different times accordingly, so the elbow’s force variability will vary the most when the flexor and extensor muscles are most active (Fig. 1(a), force on an apple). According to signal-dependent noise theory, the force during the reaching motion would be expected to vary maximally or peak at two locations (Fig. 1(b), left). To confirm this prediction, an experiment was conducted to measure the force and its variation when the elbow was flexed 50 times to reach the target [3]. Participants gripped the handle of a device that measured the elbow’s force and position. Contrary to the prediction of signal-dependent noise theory, the force-variability time-series had three local maxima and not two (Fig. 1(c), right). Therefore, this force variability cannot be explained by the previous theory. So where does the force variability come from?
3. Timing volatility as a source of movement variabilityIt has long been known that the timing of muscle activity is important in addition to its magnitude, but the effect of the timing of muscle activity on the variability of movement has not been fully investigated. In this study, we examined how the force to move a limb varies when the timing of activity of the flexor and extensor muscles is disrupted [3]. A previous study has shown that when electrical or magnetic stimulation is applied to the brain just prior to movement, the magnitude and shape of muscle activity during movement remains unchanged, and only the timing of the muscle activity is affected [4]. In other words, if brain information processing is perturbed, not only the magnitude but also the timing of muscle activity may be disrupted. Interestingly, the model predicted three local maxima in the force variability time-series during the reaching movement (Fig. 1(b), right). To test this prediction, we measured the activity of the flexor and extensor muscles during the movement and examined the relationship between the elbow’s movement variability and the timing volatility of the elbow’s flexor and extensor muscles. The timing of the activity of the flexor and extensor muscles differed significantly from trial to trial and was positively correlated with the elbow’s movement variability. The magnitude of the muscle activity also varied from movement to movement, but there was no correlation between this and the elbow’s movement variability. Thus, the experiment revealed that timing volatility is a significant factor in determining movement variability. 4. Muscles in the left and right arms have different timing volatilityAlthough it was clear that there was a correlation between the elbow muscles’ timing volatility and the elbow’s position variability, a new experiment was conducted to investigate whether a similar relationship could be observed in the left and right arms of the wrist, elbow, and shoulder joints [3]. In this experiment, the position of the handle of a device was controlled so that it would not move, and an arrow on the screen was programmed to indicate the direction of the force applied to the handle (Fig. 2(a), left). The participants were asked to push or pull the handle in the indicated direction, relying on a sound that sounded at regular intervals. The direction of the force arrow was changed depending on which joint was being tested. The force and muscle activity obtained in these experiments revealed a positive correlation between the force variability and the timing volatility of the wrist, elbow, and shoulder muscles (Fig. 2(b), right). With only right-handed participants, it was found that there was less timing volatility in the muscles of the right arm compared with the muscles of the left arm [3]. From these results, we considered the possibility that the higher accuracy of the dominant right hand may come from small timing volatility.
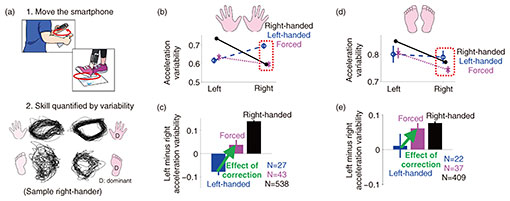
5. Relationship between dominant hand and movement varianceTo further investigate the relationship between the dominant hand and timing volatility of the muscles, data from many participants is required. However, measuring muscle activity is time-consuming and labor-intensive, making it unsuitable for large-scale experiments. To facilitate the collection of data from many participants, we devised a simple method to estimate the timing volatility of the muscles controlling a limb [3]. This method examines the movement variability during repetitive circular movements at relatively high speeds. Since circular motion is made possible by activating multiple muscles in an orderly sequence, precise timing of muscle activity is required. Because of the relatively fast and simple repetitive motion, the acceleration variability can be evaluated with only a short recording. We therefore developed an algorithm to quantify the amount of variation in acceleration trajectories when a person undergoing measurement performs a 15-second repetitive circular motion while holding a smartphone or strapping it to the leg (Fig. 3(a), upper) [3]. By expressing the difference between the three-dimensional acceleration trajectories of two consecutive cycles of cyclic motion on a distance scale for all movement cycles, the overall acceleration variability of the cyclic motion can be estimated. Participants were asked to complete the Edinburgh Handedness Inventory [5] and the Coren Footedness Inventory [6], which are conventional questionnaires used to determine the dominant hand and foot. The Edinburgh Handedness Inventory contains a total of ten items, and the respondents are asked to indicate the hand used in tasks such as writing or throwing a ball. The Edinburgh Quotient changes depending on the frequency of selecting the left hand (−1) and right hand (+1). If the Edinburgh Quotient was less than zero, the respondent was considered left-handed, and if it was greater than zero, the respondent was considered right-handed. The Coren Footedness Inventory included four items, and right-footedness was determined by asking the respondents to indicate the foot with which they kick a ball or the foot with which they take the first step when climbing up a flight of stairs. Example acceleration trajectories from both hands and feet of a right-handed participant are shown in the lower part of Fig. 3(a). Upon visual inspection, the variability in the acceleration trajectories of the dominant right limbs appears to be smaller relative to the non-dominant limbs. Using the developed algorithm, the acceleration variability can be quantified. To investigate the relationship between handedness and the acceleration variability, we evaluated the acceleration variability separately for right-handed, left-handed, and forced-handed individuals who answered that they were forced to switch from using their left hand to right hand at an early age (538 right-handers, 27 left-handers, and 43 forced-handers) (Fig. 3(b)) [3]. The right-handers showed greater variability in their left hand, while left-handers showed greater variability in their right hand. Thus, the acceleration variability of the dominant hand was smaller. Forced-handed individuals had less variability in both their left and right hands. In other words, forced use of the right hand results, on average, in a decrease in the acceleration variability of the right hand (i.e., increased dexterity), but no increase in the variability of the left hand (i.e., no decrease in dexterity). We then calculated the difference in the acceleration variability between the left and right hands of right-handed, left-handed, and forced-handed individuals (Fig. 3(c)). When left-handers were forced to use their right hand from an early age, the balance between the left and right hand’s acceleration changed considerably towards stronger right-handedness. 6. Forced use of the right hand affects foot acceleration variabilityAlthough we found that forced use of the right-hand reduced the acceleration variability of the right hand, we also examined the effect of forced correction on the feet. Foot variability was also analyzed separately for left-handed, right-handed, and forced-handed groups (Fig. 3(d)). Interestingly, right-handed individuals had, on average, less variability in their right foot (i.e., they were right-footed), whereas left-handed individuals had no difference in the acceleration variability of their left and right feet. Despite only the hand being corrected, the acceleration variability of the right foot was, on average, smaller than that of the left foot in forced-handed individuals (Fig. 3(e)). These results indicate how prolonged training of the other hand not only changes the difference in the acceleration variability between the left and right hands but also affects the acceleration variability of the feet.
7. Movement variability changes with growth and agingWe showed how prolonged training of the right-hand affects the acceleration variability of the hands and feet. We then investigated whether the acceleration variability of the limbs changes with age. Figure 4 shows the acceleration variability of the dominant and non-dominant limbs, quantified from data obtained from experiments on 608 participants ranging in age from 4 to 88. The graph shows that the acceleration variability for both dominant and non-dominant limbs decreased with growth, remained constant, and regressed with age. Although an increase or decrease in muscle strength is considered to have an effect, muscle strength is considered to increase until the 20s then begins to decline, so changes in muscle strength alone cannot explain the change in acceleration variability. Thus, changes in acceleration variability associated with growth and aging may well have to do with changes in muscle-timing volatility.
8. Conclusion and future workI described how movement variability is affected by the timing volatility of muscle activity. By developing a method to evaluate the variability of limb movements using repetitive movements, the relationship between the dominant hand and muscle-timing volatility was clarified. Previous methods were developed to examine dexterity through work rate, for example, how many pegs can be placed in a hole in a certain time or how many small blocks can be carried from one box to another. However, these methods require special equipment and are difficult to implement out in the field. Therefore, it is difficult to measure the skill of many participants from a wide age range, and it has been challenging to examine the effects of growth, aging, or individual training on the ability to control limbs. Furthermore, the ability to control the feet has been assessed using measures such as the ability to balance on one foot for 10 seconds. However, this involves not just foot control but sensory processing of whole-body motion. Therefore, such a method is not sufficient for measuring the dexterity of foot movement. We examined a method to visualize the acceleration variability by quantitatively evaluating the variability of repetitive movements over a short period. The results indicate that the acceleration variability of the dominant hand is related to smaller muscle-timing volatility. We also showed that the acceleration variability of not only the right hand but also the right foot was less than that of the left hand when the participant was forced to use their right hand from an early age. Furthermore, we found that the acceleration variability changed not only with training but also with age. The decrease in acceleration variability with growth suggests that motor learning to reduce the variability of movement progresses during growth. However, the increase in acceleration variability with age could be due to a breakdown in timing control, which may contribute to an increase in the risk of falling when walking. Although growth and aging have been shown to affect the variability of arm and leg movements, the mechanism by which the timing of muscle activity, which determines variability, is disrupted remains to be clarified. If we can identify the brain region that produces timing volatility, we will be able to clarify why timing volatility changes with growth and aging. If the mechanism and training method for decreasing acceleration variability by training with the right hand as well as growth can be elucidated, it could be applied to sports training and rehabilitation. To validate such a training method, we need a technology that can easily evaluate the movement variability of a limb. The method introduced in this article can easily enable the visualization of the acceleration variability of movements by simply moving a smartphone. We believe this has potential in sports gyms, club activities, rehabilitation facilities, and other settings, and can be used for individual training and rehabilitation. References
|
|||||||||||||