|
|||||||||
|
|
|||||||||
|
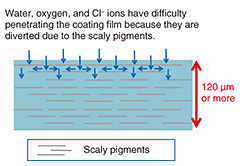
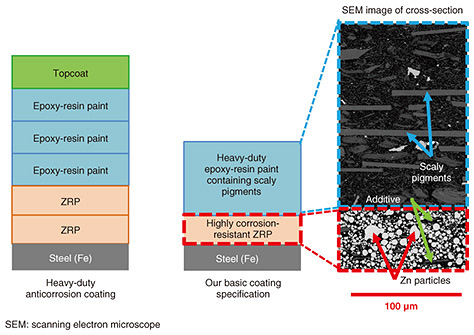
Regular Articles Vol. 23, No. 4, pp. 37–42, Apr. 2025. https://doi.org/10.53829/ntr202504ra1 Highly Corrosion-resistant Coatings for Steel Infrastructure in Coastal AreasAbstractThe NTT Group possess a huge number of outdoor telecommunications facilities composed of metallic materials or painted metallic materials, such as mobile-phone base-station towers, as part of the important infrastructure that supports telecommunications services. An increasing number of steel towers have become in need of anti-corrosion coating as their zinc layer of galvanized steel has been worn away by corrosion. Anti-corrosion coating deteriorates after 12 years or so and requires reapplying. Therefore, we are developing a long-lasting, corrosion-resistant paint to reduce the cost of repainting towers by extending the interval between reapplying anti-corrosion coating. Keywords: zinc-rich paint, gordaite, sulfate ion, calcium sulfate, cyclic corrosion test, outdoor exposure test 1. Introduction1.1 Telecommunications facilities and coatingsThe NTT Group has a huge number of outdoor telecommunications facilities (such as mobile-phone base-station towers) composed of metallic materials or painted metallic materials. Whether outdoor steel structures, such as wireless steel towers on NTT telecommunications buildings, should be repainted is determined on the basis of inspection results. Due to the many outdoor steel structures, the cost of such painting is huge. In the near future, the number of facilities that will need first-time coating is expected to increase, thus drastically increasing maintenance costs. To reduce such costs of outdoor steel structures by extending the repainting interval, highly reliable painting materials—with high resistance against corrosion and long life—must be used. 1.2 Zinc-rich paint and heavy-duty anticorrosion coatingsContaining a high concentration of Zn powder, zinc-rich paint (ZRP) has two excellent functions. First, even if the paint layer is scratched all the way to the underlying steel, the Zn corrodes preferentially in a manner that allows a corrosion-prevention current to flow through the steel to protect it (so-called sacrificial corrosion protection). Second, the Zn-corrosion product (rust) forms a dense layer that protects the damaged area of the paint layer and inhibits subsequent corrosion (so-called protective-layer function). The coating specification that includes ZRP is called “heavy-duty anticorrosion coating” and is long-lasting and resistant to corrosion. The steel to be painted is first coated with ZRP, then coated with a thick layer of epoxy-resin paint, and finally coated with a top coat of ultraviolet (UV)-resistant fluoropolymer- or polyurethane-resin paint. However, the following problems are associated with heavy-duty anticorrosion coating: the painting takes a long time due to the large number of processes (number of coats), high labor costs for painting, and high purchase cost due to the large amount of paint required. 1.3 Investigation of low-cost, process-saving coating specificationsHeavy-duty anticorrosion coating is typically applied by brush in the following steps: two coats of ZRP (each coat about 40 μm thick), three coats of epoxy-resin paint (each coat about 50 μm thick), and one coat of UV-resistant topcoat paint (about 25¡Ý50 μm thick), making a total of six coats with an overall thickness of 250 μm or more. Considering this procedure, we have been investigating ways to achieve performance similar to that of heavy-duty anticorrosion coating but at lower cost and with fewer steps. We are attempting to achieve this goal through two improvement measures. Improvement Measure 1 focuses on enhancing the barrier performance of the epoxy-resin coating and reducing degradation caused by UV radiation. Improvement Measure 2 aims to improve the corrosion-protection performance of ZRP. Improvement Measure 1 involves replacing the three coats of epoxy-resin paint and one coat of UV-resistant topcoat paint with one coat of thick-layer epoxy-resin paint containing scaly pigments. By adding scaly pigments to the epoxy-resin paint, corrosive factors such as water, oxygen, and salt can no longer travel directly through the paint toward the steel; instead, they have to bypass the scaly pigments, which has the same blocking performance against corrosion factors as the conventional (thicker) epoxy-resin paint (see Fig. 1). Epoxy-resin paint is unsuitable as the topcoat because it is sensitive to UV light. However, the addition of scaly pigments protects the epoxy resin from UV light; thus, damage by UV light is to a lesser extent than general epoxy-resin paint. By adding scaly pigments to a thick-layer epoxy-resin paint that can be applied thickly (120 μm) with a single brush stroke, we designed a paint to maintain a sufficient thickness for more than 15 years, even if the thickness is reduced slightly by UV light.
Improvement Measure 2 involves improving the anticorrosion performance of one layer (40 μm thick) of ZRP to achieve a similar performance to that of the conventional two-layer coating (80 μm thick). The goal is to achieve a similar performance (long life and corrosion resistance) to that of heavy-duty anticorrosion coating by using two layers (160 μm thick), namely, one layer of the aforementioned thick-layer epoxy-resin paint with added scaly pigments (120 μm thick) and one layer of ZRP with improved performance (40 μm thick) (see Fig. 2). The technology for improving the performance of ZRP is mainly described below.
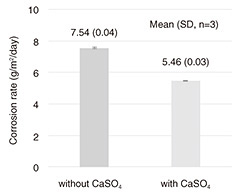
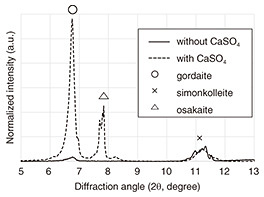
2. Improve corrosion performance of ZRP with an additiveZRP has excellent corrosion resistance even when the coating is scratched to reveal the steel (due to its previously mentioned sacrificial-corrosion protection and protective-layer function). It is therefore used in coating specifications that require high corrosion resistance and long life. Many types of Zn-corrosion products have been precipitated due to atmospheric corrosion of Zn in coastal areas, and it has been suggested that among the corrosion products precipitated, gordaite (NaZn4(SO4)(OH)6Cl·6H2O) has an excellent protective-layer function [1]. We have been attempting to improve the corrosion resistance of ZRP in coastal areas, which are particularly susceptible to severe corrosion, by intentionally promoting the precipitation of a large amount of gordaite in the Zn rust that forms when the Zn in ZRP corrodes [2]. With a pH of around 8.1, seawater is slightly alkaline and rich in both sodium (Na+) and chloride (Cl-) ions. It also contains sulfate (SO42-) ions at molar a concentration one order of magnitude lower than that of Cl- ions. When Zn is corroded by airborne sea salt, the main precipitated-corrosion products that contain chlorine are simonkolleite (Zn5Cl2(OH)8·H2O) and gordaite. However, we assume that by adding SO42-, it would be possible to precipitate a higher proportion of gordaite. 2.1 Selection of additive sulfateIf a sulfate with excessive water solubility is added, the sulfate present in the exposed parts of the ZRP will be dissolved quickly and lose its ability to supply SO42- in a short time. Thus, the parts of the ZRP where the sulfate has been dissolved away will become voids, which may reduce the paint’s ability to block corrosion factors. Conversely, sulfate with insufficient water solubility cannot supply SO42- ions to a concentration sufficient to increase the precipitation rate of gordaite. Among the common sulfates that are practical to add to paints, calcium sulfate (CaSO4), which has moderate water solubility, is safe and inexpensive, so we selected it as the additive. 2.2 Confirmation of enhanced gordaite formation and improved corrosion resistance by using Zn platesBefore adding CaSO4 to ZRP, we confirmed the effect of adding it by using Zn plates. By conducting a cyclic corrosion test (CCT), an accelerated corrosion test that repeats the steps “salt spray,” “drying,” and “wetting,” we compared the results of corroding a Zn plate in salt water with or without added CaSO4. The test program and salt water (test solution) used for the CCT were our proprietary test program and test solution [3][4] that can quickly reproduce atmospheric corrosion of Zn in coastal areas. According to the results of the CCT (Fig. 3), when Zn was corroded in test solution containing CaSO4, the corrosion rate of the Zn plate was reduced by approximately 30%. X-ray-diffraction analysis of the Zn-corrosion products confirmed that the ratio of gordaite to simonkolleite significantly increased (Fig. 4).
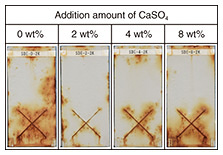
2.3 Confirmation of the effect of CaSO4 additive on improving ZRP performanceSamples of SS400 steel plates (after blasting) were corroded by conducting the CCT, and the CCT-induced rust was removed (in accordance with quality ISO 8501-1-St3) to produce rust-free steel plates. To prepare painted samples, the steel plates were then painted with CaSO4-added (commercially available) ZRP. The painted samples were scratched (with a utility knife) down to the steel and subjected to CCT and outdoor-exposure tests on the coast of Miyakojima, Okinawa Prefecture. According to the results of the CCT (Fig. 5), corrosion resistance of the samples containing 1- to 16-wt% CaSO4 notably improved, while corrosion resistance of the sample containing 32-wt% CaSO4 decreased. According the results of the outdoor-exposure tests (Fig. 6), corrosion resistance of the 2-wt%-CaSO4-added sample appears to be the best, and corrosion resistance of the 4- to 8-wt%-CaSO4-added samples decreased according to the increased amount of CaSO4 additive.
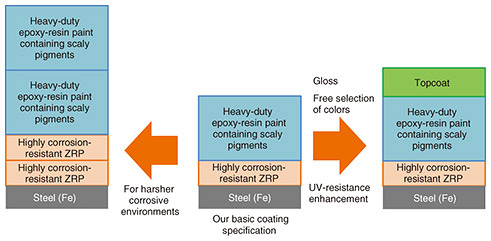
The optimal amount of CaSO4 additive slightly differed between the CCT and outdoor-exposure test (it was less in the latter case). This discrepancy in the test results is explained as follows. The CCT period (just under three months) ended before the CaSO4 in the paint was completely dissolved. During the latter half of the two-year outdoor-exposure test, however, most of the CaSO4 in the coating layer had dissolved, and as more CaSO4 was added to the sample, more voids appeared in the paint coating, which degrades the effectiveness of corrosion prevention. However, the effect of CaSO4 additive remained for two years, even in the extremely harsh environment of the Miyakojima coast, which is hot, humid, and rainy, and where the paint is directly exposed to sea spray during strong winds. Therefore, in milder environments, it is expected that the progression of corrosion will decrease for several years compared with that of regular ZRP without CaSO4 additive, even after the paint layer is scratched to the steel. 3. Future prospectsAs explained above, our goal is to achieve a similar performance (long life and corrosion resistance) to that of heavy-duty anti-corrosion coating by using two layers, namely, one layer of the aforementioned thick-layer epoxy-resin paint with added scaly pigments and one layer of ZRP with improved performance. To select the optimum coating specification flexibly according to the installation environment and type of steel structure, we are comparing and examining various coating specifications on the basis of the basic specification shown in the middle of Fig. 2. For example, the basic specification has a top layer of thick epoxy-resin paint containing scaly pigments; however, this coating specification has a limited selection of colors (without gloss). Therefore, if gloss or free selection of colors is required, it is necessary to add another layer of topcoat paint on top of the basic specification. Although the scaly-pigment-added thick-layer epoxy-resin paint is somewhat resistant to UV light (thanks to the scaly pigments), over a long period of 15 years or more, the paint’s anti-corrosion performance is expected to decrease as the paint-layer thickness decreases. Therefore, to increase the repainting interval, it is advisable to add a topcoat made of a resin that is similarly resistant to UV light (Fig. 7, right). Although the basic specification is sufficient to withstand corrosion in most coastal areas, in particularly harsh environments, such as close to the ocean, it may be necessary to apply twice the number of coats (with double the thickness) of ZRP or thick-layer epoxy-resin paint with scaly-pigment additive (Fig. 7, left). To be able to select the most-suitable coating specification while taking into account the cost-effectiveness of each coating specification, we are comparing and verifying various coating specifications through CCT and outdoor-exposure tests on the coast of Miyakojima, Okinawa Prefecture.
References
|
|||||||||