|
|||||||||
|
|
|||||||||
|
Special Feature: Applied Technology for Millimeter and Terahertz Electromagnetic Waves Vol. 7, No. 3, pp. 35–39, Mar. 2009. https://doi.org/10.53829/ntr200903sf6 Terahertz Spectroscopy Technology for Molecular NetworksAbstractIn this article, we introduce the latest terahertz spectroscopy technology. Recent advances such as ultrashort pulsed lasers have dramatically improved measurement time and sensitivity, making it possible to investigate phenomena, such as molecular networks, which have previously been difficult to study. The last few years have seen rapid progress in terahertz spectroscopy, with accomplishments in the life-science and biotechnology fields, such as new types of medical imaging, that promise to contribute to a safer and more secure society.
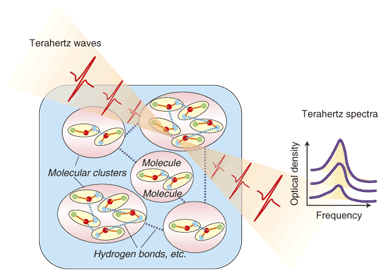
1. Terahertz spectroscopy and molecular networksTerahertz waves have frequencies in the range from 0.3 to 3 THz (corresponding to wavelengths from 100 to 1000 µm) and are promising for safe, non-damaging inspection applications because they have linear propagation characteristics and can penetrate materials such as plastic, paper, rubber, wood, and ceramics. Terahertz spectroscopy has also been attracting attention because of rapid advances in technology for generating and detecting terahertz waves, and researchers are pursuing the possibility of new chemical sensing methods. Because of their high resolving power compared with waves in the gigahertz band, terahertz waves also offer better spatial resolution in imaging and higher data transmission speeds in wireless communications. Other fields with possible applications include information technology (wireless communications), agriculture and food products, security (detecting concealed objects, dangerous substances, etc.), biomedical, environmental and space instrumentation, and industry (LSI (large-scale integration) fault analysis, new materials development, etc.). Imaging methods using the penetrating abilities of terahertz waves have fundamental differences from X-ray imaging in that they are safe and can use the vibration of molecules to identify specific materials. Applications such as pathological examination of tissues or identification of drugs or explosives in postal packages have received attention. In particular, the 1–3-THz band corresponds to the oscillations of hydrogen bonds and other forces between molecules and to phonon modes in crystal lattices. This is promising for identifying toxic or explosive substances and also has applications in the spectral analysis of macromolecules such as proteins. Molecules tend to form clusters based on the weak forces acting between them, such as hydrogen bonds and van der Waals forces (Fig. 1). In these clusters, protein and water molecules attach to medication molecules and form large molecular networks. These networks are a very important factor related to issues in the life-science and biotechnology fields, such as the effectiveness of medications and the high-order structure of proteins, but they are still not fully understood. One reason for this is that the cluster size is on the nanometer order, and there are few ways to measure the size when the water component of a cluster is included. It has been known for some time that the resonant frequencies of these molecular networks are in the terahertz range, but it has been difficult to make far-infrared absorption spectrum measurements at frequencies below 6 THz (approx. 200 cm-1), which corresponds to room temperature. It has recently become possible to perform stable measurements of 0.17-THz spectra, however, owing to developments in terahertz time-domain spectroscopy (THz-TDS), which uses terahertz electromagnetic pulses on the order of 10 fs generated using an ultrashort-pulse laser.
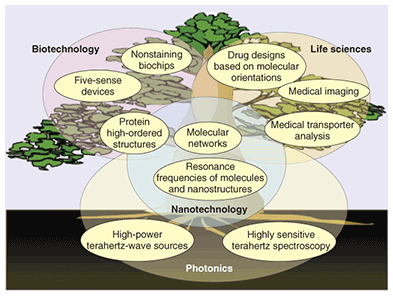
Terahertz spectroscopy is being developed with support from optical technologies, such as high-output light sources and high-sensitivity detectors (Fig. 2). Interaction among molecules and resonant frequencies between them are being actively explored, and topics like the crystal polymorphisms of medications are becoming key applications for terahertz spectroscopy. The development of terahertz spectroscopy has just begun, but if we can improve the spectral sensitivity and develop a theory explaining the interaction between molecules and terahertz radiation, including simulation, then many new applications will become possible. In biotechnology, these include ways to determine the high-order structure of proteins or new devices that mimic all five human senses. In the life sciences, technology for controlling the position of molecules could lead to the discovery and design of new drugs or to new medical imaging techniques.
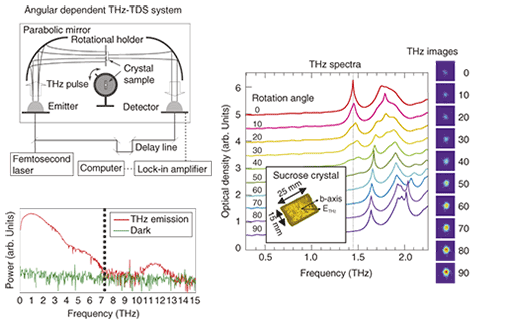
2. Analysis of hydrogen-bond network arrangementIn the crystals of sugars or amino acids, the hydrogen-bond network forming the crystal determines the orientation of the entire molecule. As a familiar example, let us consider single sugar crystals (rock candy). We developed the angle-dependent THz-TDS system shown in Fig. 3 to study hydrogen-bond networks. A terahertz pulse is generated using an ultrashort-pulse laser and photoconducting antenna. A similar photoconducting antenna is used for the detector. Although frequencies up to 14 THz are generated, those from 7–10 THz are absorbed by the semiconducting material of the photoconducting antenna, so the effective measurement range is from 0.1 to 7 THz.
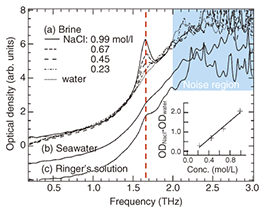
The terahertz spectrum of the sucrose crystal was measured while the incident angle was changed. We set the angle to be 0° when the polarization of the terahertz radiation was parallel to the crystal’s b-axis as determined by X-ray diffraction. The spectrum’s shape changed significantly as the angle was rotated from 0° to 90°. In particular, the 1.45-THz peak was very large at 0°, but it completely disappeared at 90°, when the incident polarization was parallel to the b-axis. In contrast, we found other peaks that were large at 90°. This indicates that there are several hydrogen-bond networks in a single sucrose crystal and that when they are oriented parallel to the polarization of the incident radiation, peaks in the spectrum appear when dipoles absorb the terahertz radiation. The terahertz images in Fig. 3 show the resulting transmission images when 1.45-THz laser light was directed at the single sucrose crystal at an angle from 0° (top image) to 90° (bottom image). At 0°, the sucrose crystal absorbed the terahertz laser radiation, so there was no transmission image, but at 90°, there was a clear image because the radiation was not absorbed. In other words, the intensity of certain frequencies could be controlled by changing the angle of the crystal. This indicates that it should be possible to design optical materials for the terahertz band by making use of the absorption characteristics of molecular networks. 3. Measurement of ions in iceSodium chloride (NaCl) is one of the basic materials in life forms, and it exists in the bodies and structures of many organisms. There are many electrochemical and other highly sensitive methods for detecting Na+ and Cl– ions in solution, but these ions are difficult to detect when they are diffused in ice. Here, we introduce a THz-TDS-based method we have discovered for measuring the amount of NaCl dissolved in ice without melting it. The results of cooling some NaCl solutions of various concentrations to 123 K to freeze them and taking THz-TDS measurements of them are shown in Fig. 4. A sharp peak was observed at 1.66 THz. The intensity of this peak was proportional to the NaCl concentration, indicating that the peak can act as an NaCl fingerprint. In this way, we discovered that a frozen solution of a simple substance could have a fingerprint absorption spectrum in the terahertz range. However, with current terahertz spectral analysis technology, it is very difficult to assign the peak. It was not observed in measurements of the terahertz spectra of NaCl crystals, so it cannot be attributed to the combination of Na+ and Cl– ions. Furthermore, no such characteristic peak was detected in the 0.2–2.0-THz range in similar measurements of samples of LiCl and KCl, which have different cations. In similar measurements of NaBr and NaI, which have different anions, several peaks at different frequencies were observed. From these results, we conclude that the NaCl fingerprint absorption peaks are related to the molecular network formed by the Na+ and Cl– ions in the hydrated state.
To confirm the usefulness of this method for detecting NaCl, we also measured frozen samples of seawater (2.6% NaCl) and Ringer’s solution (0.86% NaCl, which has electrolytic properties similar to extracellular fluid): similar peaks were observed near 1.66 THz. To describe this phenomenon more accurately, it will be necessary to perform measurements on more types of salt and provide support in the form of theoretical calculations. 4. ConclusionWe have introduced examples of NTT’s research on molecular networks using terahertz spectroscopy. To establish this technology as a new spectral analysis method, future research will need to focus on improving device technology, increasing the power of light sources and the sensitivity and speed of detectors, improving realtime imaging technology, and solving various other issues like the theoretical attribution of terahertz spectral peaks and construction of a database. Terahertz spectroscopy is very promising as a tool that will be available in the near future for a wide variety of applications, such as medical imaging and bio-chips, in the life-science and biotechnology fields. AcknowledgmentWe thank Dr. Rakchanok Rungsawang (currently at Cambridge University, UK) for fruitful discussions about terahertz spectroscopy. References
|
|||||||||