|
|||||||||||||||||||
|
|
|||||||||||||||||||
|
Special Feature: Nanobiotechnology Research Vol. 7, No. 8, pp. 10–14, Aug. 2009. https://doi.org/10.53829/ntr200908sf2 Reconstitution of Membrane Proteins into Lipid Bilayer and Its Application to NanobiodevicesAbstractIn this article, we introduce the reconstitution of membrane proteins into lipid bilayers and describe the challenge of creating nanobiodevices based on our fundamental technologies.
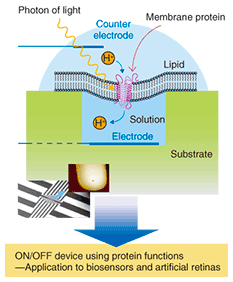
1. IntroductionRecent progress in the fields of nanofabrication and nanometer-scale measurement has attracted attention to a new research field that is a fusion of nanotechnology and biotechnology and that has a huge potential market. In this combined field, it will be possible to utilize nanotechnology as a tool for diagnostics and the medical treatment of diseases or for the examination of new biological functions as well as for creating nanobiodevices, which are nanometer-scale devices that provide highly efficient biological functions. Therefore, these nanobiodevices have attracted interest as a new research field in relation to medicine, pharmaceuticals, and science because they can provide state-of-the-art biological functions. Membrane proteins are proteins that function when they attach to or pierce lipid biological membranes. They account for half the weight of a cellular membrane. There are several different membrane proteins: transmembrane proteins such as receptor proteins, which accept extracellular signals and transfer them into cells; transporters, which transfer ions by using biological energy; and peripheral membrane proteins such as adhesive proteins, which control cell adhesion. Receptor proteins are classified as ion-channel type (ionotropic) receptors and G-protein coupled (metabotropic) receptors. An ion-channel type receptor changes its structure by ligand binding, allowing ions to flow into the cells by opening an ion channel, and transfers signals by inducing changes in electrical potential. Biological signaling is essential for maintaining life, and disorder can induce diseases. Thus, receptor proteins are highly functional, ultrasmall devices related to biological functions. As one example of a nanobiodevice, NTT Basic Research Laboratories is studying a sensor chip that utilizes receptor protein functions (Fig. 1). By reconstituting purified receptor proteins into an artificial lipid membrane, we hope to obtain ones that function as receptor proteins in vivo. Moreover, by controlling the number and species of receptor proteins, we should be able to achieve multi-functional state-of-the-art nanobiodevices. If an ultrasmall device with a switching function using biological molecules can be achieved, it will be possible to create very small sensors and assist biological functions by implanting them in the human body.
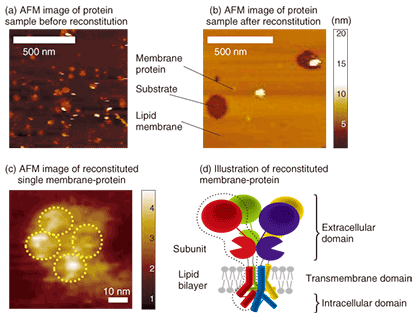
At NTT Basic Research Laboratories, we focus on biologically functioning molecules, namely receptor proteins, with the aim of creating nanobiodevices. To make nanobiodevices, we must combine and integrate biological molecules such as proteins whose size is about 10 nm. This requires several fundamental technologies, for example, the manipulation and observation of molecules, control of molecular alignment and orientation, reconstitution of membrane proteins into a lipid, maintenance of the molecular function, and selection of biocompatible materials. 2. Structural examination of reconstituted receptor protein2.1 Purification and reconstitutionReceptor proteins can be purified from organs or cultivated cells. Purification has different steps: first the organs or cells are homogenized, then a membrane fraction is obtained using ultracentrifugation, and finally the membrane fraction is solubilized using detergent. All the purification processes proceed better at 4°C. The solubilized fraction is then purified using several different columns. The details differ depending on the protein. Most receptor proteins, which are membrane proteins, consist of several different subunits. The subunits usually consist mainly of three domains: the extracellular, intracellular, and transmembrane domains. Receptor proteins can be orientated in the same direction and can function as a result of the reconstitution of the lipid membrane. There are several reconstitution methods [1], but they are all based on the same idea: mix the protein well with lipid and detergent and remove the detergent gradually. This method produces proteoliposomes that are liposomes with reconstituted receptor proteins. These are lipid bilayer spheres on which receptor proteins are reconstituted. These spherical proteoliposomes fuse and form a planar lipid bilayer with reconstituted receptor proteins on a substrate or on structures that are suitable for device application. 2.2 ObservationThere have been many studies on the structural observation of receptor proteins. Crystallographic analysis is mainly performed using X-ray analysis, while single protein observation is mainly undertaken using cryo-electron microscopy [2]–[4]. These observation methods have extremely high spatial resolution, but they are unsuitable for continuous structural observation of functioning proteins. Atomic force microscopy (AFM) can resolve a single protein molecule as small as 10–20 nm in liquid. Therefore, this method is different from the abovementioned ones in terms of its ability to reveal the structure and dynamics of single protein molecules under physiological conditions [5]. AFM observation of protein structure has been studied since the late 1990s by Engel et al. and other groups [6]. A protein sample after purification and one after purification & reconstitution observed by AFM are shown in Figs. 2(a) and (b), respectively. The pre-reconstitution sample on a mica substrate (a) exhibits small flat domains with a height of 4 nm (dark yellow) and higher structures (white) on the substrate (dark brown). After reconstitution (Fig. 2(b)), we can see a large flat domain with a height of 4 nm (dark yellow) and 2–10-nm-high dots with a diameter of several tens of nanometers (white). The flat 4-nm-high domains are a lipid bilayer and the white structures are membrane proteins. The proteins inside the large lipid bilayer were revealed by the reconstitution. This result showing proteins of several different sizes suggests that the proteins occur both singly and in aggregations. The antibody reaction confirmed that a single receptor-protein has a height of 2–3 nm from the lipid bilayer and a lateral dimension of 20–30 nm. We then observed the structure of the single 2–3-nm-high protein.
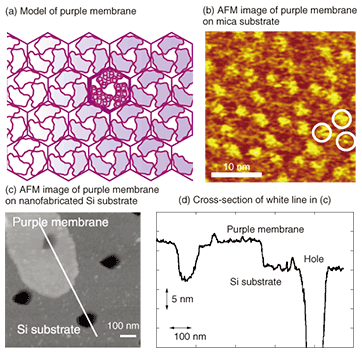
AFM observation of a single receptor-protein (membrane protein) revealed four objects on the flat lipid bilayer, as shown in Fig. 2(c). Since this receptor protein has a tetrameric structure with four subunits, as shown schematically in Fig. 2(d), the AFM image shows all four subunits of this protein. The observed tetrameric structure was expected to be the top view of the extracellular domain of the receptor protein reconstituted into a lipid bilayer. Because AFM enables us to observe functioning single receptor-proteins, we can expect to determine the relationship between function and structure in the future. 3. NanobiodevicesTo make the nanobiodevice shown in Fig. 1, we must locate a lipid membrane with membrane proteins so that it covers the nanoscale holes. We also require AFM observation of the nanohole with resolution on the level of a single molecule to confirm that the membrane proteins are located in the device structure as designed. An AFM observation result for a purple membrane suspended over a 100-nm hole on a Si substrate is shown in Fig. 3. The purple membrane is a lipid bilayer membrane consisting of a membrane protein, Bacteriorhodopsin, which is a light sensitive proton pump. It is similar to the membrane protein in the retina and is expected to be used as a material for light-sensitive biodevices or artificial retinas.
In a lipid membrane, the proteins form a trimer, which constructs a two-dimensional crystal with a hexagonal shape, as shown in Figs. 3(a) and (b). The purple membrane adsorbed on the Si substrate covering one of the holes is shown in Fig. 3(c). A cross-sectional view (Fig. 3(d)) indicates that the AFM cantilever pushes and bends the purple membrane during the observation, but the membrane completely covers one hole and thus completely separates the inside of the hole from the outside. Although biological membranes including the purple membrane are very soft and fluid, it is possible to observe membrane proteins on nanoscale device structures by selecting AFM observation conditions that reduce the force exerted by the cantilever on the samples. Here, we discuss a purple membrane, which is a protein that forms a two-dimensional crystal, but we can expect to be able to observe and examine the function of receptor proteins reconstituted into the suspended lipid membrane. The lipid bilayer that covers small holes as discussed above can be considered to be a cell membrane. AFM is usually used to observe samples fixed firmly on a substrate, but this method enables us to observe the proteins in a membrane without making contact with the substrate under physiological conditions. It is possible to perform a quantification examination of the mechanical properties of the membrane itself [7]. By changing the solutions inside and outside the hole separated by the lipid membrane, we can create a device environment that is similar to that in vivo. We can expect to progress toward an understanding of the relationship between the structure and function of membrane proteins if we succeed in achieving both structural analysis using AFM and functional analysis using electrophysiological or optical measurement in such an experimental environment. 4. ConclusionNTT Basic Research Laboratories is undertaking projects designed to create devices that use the receptor protein function. This research is being performed partly in collaboration with the University of Oxford. AFM observation of a single receptor-protein allows the structural observation of functioning receptor proteins in liquid and is expected to reveal the details of the receptor protein response characteristics and structural changes. Nanobiodevices based on functioning receptor proteins should enable us to construct ultrasmall sensors. Neurotransmitter sensors could be used for clinical applications to alter perception by implanting them in the body. Thus, the use of receptor proteins in device fabrication requires several fundamental technologies. At NTT Basic Research Laboratories, we are studying other technologies such as ways to control the protein arrays and simultaneously transport proteins to arbitrary positions [8]. Research combining these technologies is expected to develop further. References
|
|||||||||||||||||||