|
|||||||||||||
|
|
|||||||||||||
|
Special Feature: Nanobiotechnology Research Vol. 7, No. 8, pp. 20–24, Aug. 2009. https://doi.org/10.53829/ntr200908sf4 Artificial Cell Membrane on Patterned Surface––Growth Control and Microchannel Device ApplicationAbstractThis article reviews a technique for growing a lipid bilayer, which is an artificial cell membrane, on solid surfaces such as a silicon wafer (a semiconductor) and glass (an insulator). This technique is based on the self-spreading phenomenon whereby lipid molecules spontaneously form a supported lipid bilayer at a solid-liquid interface. We present a way of achieving positional and directional control of a self-spreading lipid bilayer by using surface patterns. We also describe a new type of microchannel device using the self-spreading lipid bilayer as a molecule carrier and report fluorescence resonance energy transfer observations.
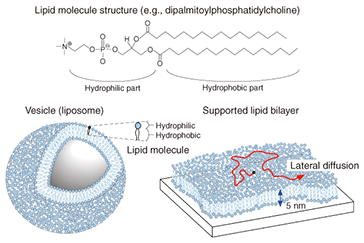
1. IntroductionWhat is the smallest unit of life? One possible answer is a cell. Every cell has a pair of deoxyribonucleic acid (DNA) molecules (double helix) containing all the information needed for building a living thing. We often hear that a DNA test on a hair left at the scene of a crime has provided proof of an offender’s identity. Furthermore, cloning techniques that were once only science fiction are now widely available. In plants and animals, including vertebrates and unicellular animals, each nerve and skin cell has a membrane that demarcates the inside of the cell from the outside. We can imagine that the softness or hardness of a cell depends on its type. However, the basic structure of the membrane is unique. It is called a lipid bilayer (Fig. 1). The basic component of the membrane is a lipid molecule, which is an amphiphilic molecule containing both a hydrophilic part and a hydrophobic part. This lipid molecule is not soluble in water, but forms gigantic self-organizing structures called vesicles or liposomes. The hydrophilic and hydrophobic parts have characteristics similar to water and oil, respectively. The vesicle structure is stable in an aqueous medium because the hydrophobic part is not exposed to the aqueous medium. The bilayer structure is formed by two monolayers so that their hydrophobic planes face each other. The membrane structure of the vesicle, namely the lipid bilayer structure, is identical to the basic structure of a cell membrane. Thus, we call the lipid bilayer an artificial cell membrane.
Lipid molecules can be extracted from egg yolk (for example) or obtained by chemical synthesis. A variety of dye-conjugated lipid molecules are commercially available, and some of them were used for the experiments described in this review. 2. Supported lipid bilayerAn artificial cell membrane can exist stably both in vesicle form and as a planar structure on a solid surface, which is called a supported lipid bilayer (SLB). Although an SLB is a membrane only 5 nm thick, it can be easily observed using a fluorescence microscope. However, to facilitate observation, one must incorporate a small number of dye-conjugated lipid molecules into the fabricated SLB. The dye provides visible fluorescence when excited by light at a specific wavelength. Typical observations are shown in Fig. 2. In this case, a synthetic lipid molecule containing fluorescein, a dye yielding green fluorescence when excited with a 488-nm laser, was mixed into a lipid extracted from egg yolk. One characteristic of the artificial cell membrane is lateral diffusion. This is a very interesting property whereby the lipid molecules forming the membrane are always moving randomly within the membrane: this is the origin of the dynamic activity of life. The lateral diffusion is maintained for the SLB. If we focus on one molecule, we see that it moves randomly in a manner like that indicated by the red arrow in Fig. 1 (bottom). This was experimentally confirmed by the results shown in Fig. 2. When exposed to high-power laser light irradiation, the fluorescein molecules were fatally damaged and lost their luminescence. Soon after irradiation of the circular area in the center of the observation area, a dark circle appeared (t = 0). Over time, this area gradually recovered and became fluorescent again. This is because the nonluminescent dye-conjugated molecules mixed with the neighboring fluorescent molecules by lateral diffusion. This proves that the SLB has fluidic liquid characteristics. This can be understood from the following example: a drop of black ink dropped into a cup of thin green liquid will diffuse and, after a period of time, the liquid will become homogeneously green.
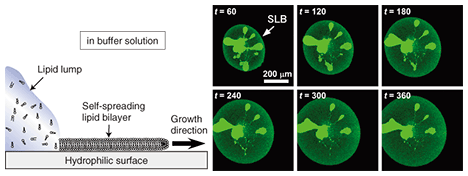
3. Self-spreading: Artificial cell membrane growthAlthough there are several known methods for preparing an SLB, we focus on self-spreading [1], which is schematically illustrated in Fig. 3. Self-spreading is a continuous self-organization process whereby an SLB is formed at a solid-liquid interface by lipid molecules supplied from a lipid source. It is surprising that the complex and highly ordered SLB structure is formed spontaneously by lipid molecules that are randomly oriented in the source. It shows us the dynamism of life where the formation of highly ordered structures is driven by molecular self-organization.
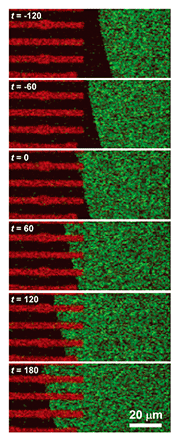
The following practical experiments were performed. A chloroform solution of dye-conjugated lipid (1–5 mol%) in egg-yolk-extracted lipid was prepared. The chloroform was then evaporated to yield a sticky solid lipid mixture. A small amount of this solid, which acts as a lipid source, was affixed to a solid surface. Tests were performed on both a silicon wafer (a semiconductor) and on glass (an insulator): both yielded the same results. Self-spreading was initiated by immersing the surface gently in a buffer solution. Typical SLB growth observations are shown in Fig. 3. It is clear that the enclosing circle, which exhibited green fluorescence, expanded over time. This large circle is an SLB and the brighter areas near the center of the circle are the lipid source. The growth speed exceeded 10 µm min–1 in the initial stage of self-spreading. When we observe this directly through the microscope, it gives us the impression that the SLB is alive, which is why I use the word grow for the SLB in this article. 4. Control of membrane growthOur next goal was to control the position and direction of the SLB grown on the surface [2], [3]. As mentioned above, both sides of the cell membrane are hydrophilic surfaces in aqueous media. With a self-spreading SLB, one side faces an aqueous medium and the other side faces a solid surface. The solid surface used in Fig. 1 was in fact a hydrophilic surface, which is why the SLB was stably formed on the surface. This led us to imagine that self-spreading cannot occur on a hydrophobic surface. Hydrophilic and hydrophobic surfaces, including the pattern they form, can be prepared easily. In order to observe the self-spreading behavior on such a patterned surface, we fabricated a hydrophobic area by using photoresist on a hydrophilic SiO2 wafer. The results are shown in Fig. 4 [2]. The photoresist pattern can be seen in red when excited by a 488-nm laser, whereas the SiO2 surface did not become fluorescent. Similar to Fig. 3, SLB growth with fluorescein yielding green fluorescence was observed over time. After the front edge of the SLB reached the pattern, the SLB continued self-spreading on only the hydrophilic surface. This means that we can control the self-spreading position freely by using surface patterns.
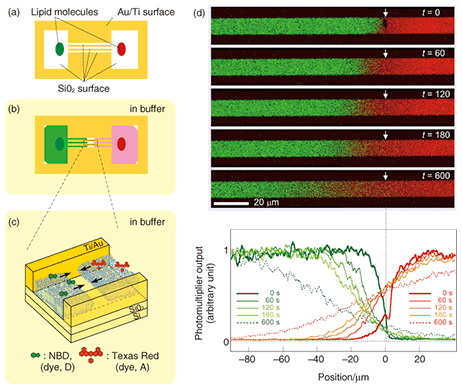
5. Transportation of molecules by SLB: Application to a new type of microchannel deviceIf we look at experimental results, such as the example observations obtained with a fluorescence microscope in Figs. 3 and 4, from a different viewpoint, we can understand that the dye molecules embedded in the SLB are being transported along the pattern by self-spreading. This idea led us to propose a new type of microchannel device using a self-spreading SLB as a molecule carrier [2], [4]. The device’s operation is simple: (1) different molecules are transported by different SLBs from both sides of the straight pattern by self-spreading and (2) the molecules mix within a unified SLB by lateral diffusion after the two SLBs have collided. The principle is similar to that of a microchannel device recently used to analyze very small amounts of materials. A schematic illustration of the device is shown in Fig. 5. Our device typically has straight lines that are 10 µm wide and 400 µm long and bridging two 500-µm squares. The pattern can be fabricated by using Au on a quartz wafer by the conventional lift-off process. Two lipid sources containing different dye-conjugated lipids (NBD and Texas Red) are placed inside squares (each in a different square). The substrate is then gently immersed in a buffer solution to initiate self-spreading. After a certain period of time, the self-spreading SLBs approach each other and finally collide. After the collision, the two SLBs are unified and the self-spreading ceases. As the unified SLB possesses lateral diffusion characteristics, the transported molecules are now mixed with each other within the membrane.
Using our device, we have successfully observed a fluorescence resonance energy transfer (FRET). This is a phenomenon whereby energy transfers from a dye molecule (called the donor (D)) to another kind of dye molecule (called the acceptor (A)). We clearly observed green (D) and red (A) fluorescence in each region in Fig. 5 before the collision. After the collision, D and A were diffused symmetrically with respect to the point of collision into the other region. Thus, the distribution of a diffused dye-conjugated lipid must be symmetric in relation to the point of collision. Correspondingly, we observed red fluorescence from A. However, green fluorescence from D could be observed only in the vicinity of the collision point and not in the diffusion region on the positive side of the abscissa in Fig. 5. This indicates that an excited donor in this region does not lose its energy by emitting fluorescence, but via the FRET process. Our experiments have also revealed the FRET mechanism. In fact, we have succeeded in performing a quantitative determination of the dependence of the FRET efficiency on the D-to-A ratio or D-to-A distance. Because the FRET efficiency is high only when the D-to-A distance is very short (less than 10 nm), FRET has recently been used for the single molecular-level observation of membrane proteins. The results obtained from our original microchannel device will be useful for understanding these biological experiments. 6. Future perspectiveOur microchannel device will make possible chemical analyses that use very few molecules, and hopefully only a single molecule in future. To develop the device further, it is necessary to test many new molecules for transportation by our system. It is especially important to be able to transport the membrane proteins that play an important role in information signal exchange in the nerve cell system. On the other hand, physical and chemical research using an SLB is also attracting much attention. NTT Basic Research Laboratories has a great advantage in this new field of research thanks to a unique idea and a state-of-the-art nanostructure fabrication technique that have recently been reported [5]. References
|
|||||||||||||