|
|||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||
|
Special Feature: Nanobiotechnology Research Vol. 7, No. 8, pp. 36–40, Aug. 2009. https://doi.org/10.53829/ntr200908sf7 Neural Function Observation with Microelectrode ArrayAbstractWe show that a polymerized microelectrode array (MEA) can act as an interface with a neural network. A blend of poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) (PEDOT/PSS) and ethylenedioxythiophene (EDOT) was electrochemically polymerized on indium tin oxide (ITO) microelectrodes. Dissociated cortical neurons were plated onto the MEA and cultivated. We measured their spontaneous activity and confirmed that a neural network could be formed on the MEA. We then evoked a network response much greater than that obtained with bare ITO microelectrodes. A conducting polymer is a suitable material for an MEA designed to measure and evoke neural activity because its low impedance allows it to measure smaller signals and evoke greater responses.
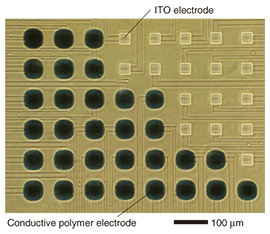
1. IntroductionThe nervous system of vertebrates can be divided into the peripheral and central nervous systems. The peripheral nervous system can be subdivided into the afferent and efferent nervous systems. The afferent nervous system transmits sensory information concerning the five senses (touch, taste, hearing, sight, and smell). This sensory information is received by the central nervous system, which processes it for learning and memory, and/or provides the efferent nervous systems with motor information. The efferent nervous system transmits motor information to the limbs for motor control. The nervous system has two mechanisms for transmitting information: current flow involving ions migrating across the cell membrane in one neuron and chemical diffusion through a synapse between pre- and postsynaptic neurons. Chemicals are neurotransmitters that excite or inhibit the activity of postsynaptic neurons. Therefore, the measurement or use of electrical or chemical signals for a brain-machine interface is a fundamental technology. In this article, we introduce an electrochemically polymerized microelectrode array (MEA) for use as a brain-machine interface. 2. Electrochemical polymerization of microelectrodesConductive polymer can be synthesized electrochemically or chemically and should let us break through the limits of silicon technologies. The significance of this was highlighted by the awarding of the 2000 Nobel Prize in Chemistry to Hideki Shirakawa of the University of Tsukuba (shared with Alan Heeger and Alan MacDiarmid) for the discovery and development of conductive polymers [1]–[4]. We have fabricated a planar MEA that we polymerized electrochemically from poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) (PEDOT/PSS) and ethylenedioxythiophene (EDOT) on indium tin oxide (ITO) electrodes, as shown in Fig. 1 [5]. When a polymerized MEA is used to measure neural activity in an electrolytic solution used as a culture medium, there are two kinds of charge transportation. One is the transportation of a positively charged ion and an electron between the conductive polymer and the electrolytic solution in accordance with the reaction (PEDOTn+)nPSS–+ne–nC+(solution) ⇔(PEDOT)nPSS–nC+, where C+ and e– represent a cation and electron, respectively [6], [7]. The other kind of charge transportation occurs in the conductive polymer itself. Conductive polymers act as electrical semiconductors whose electrical conductivity falls in the range between that of a conductor and an insulator. This conductivity increases progressively with acceptor or donor doping. With PSS doping, PEDOT becomes a p-type material. Moreover, its impedance can be decreased because of its porosity. An electrode with low impedance improves the signal-to-noise ratio and makes it possible to measure smaller signals with a polymerized MEA.
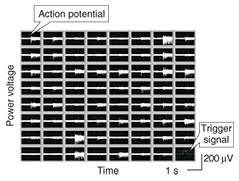
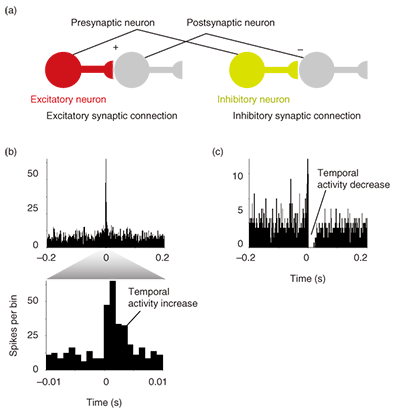
3. Electrical measurement of neural activityInformation processing in the central nervous system is based on the interaction between excitatory and inhibitory neurons. Since the development of the MEA, it has been possible to observe long-term neural dynamics in dissociated cell cultures [8]–[11]. A high-density MEA consisting of small electrodes is suitable for the parallel, spatiotemporal measurement of neural activity. However, small electrodes reduce the signal-to-noise ratio as a result of their high impedance, so we developed an electrically polymerized MEA to reduce the impedance of microelectrodes. We increased the contact area with the electrolyte by creating a rough electrode surface and estimated the stimulation and recording properties of the MEA when used as an interface with a neural network. In this section, we show that a polymerized MEA can be used to measure the interaction between the excitatory and inhibitory neural activities of dissociated cultures on the MEA. The electrophysiological signal was measured with an MEA-based neural recording system [12]. Spontaneous activities were detected as spiked waveforms from electrophysiological signals when the amplitude was below the -5 sigma noise level (Fig. 2). The detected spontaneous activities were clustered by using principal component analysis [13]. We then calculated cross-correlograms with a 1-ms bin width (total width: ±0.2 s) between two clustered spontaneous activities to identify the existence of a synaptic connection. Correlation was mainly classified into positive and negative groups on the basis of the type of excitatory and inhibitory synaptic connections, as shown in Fig. 3(a). The cross-correlograms in Fig. 3(b) exhibit positive correlation with an increase in temporal activity. This means that one clustered spontaneous activity is followed by another with an axonal conductive delay and a synaptic delay. This is shown by the observation that two neurons were linked by an excitatory connection (Fig. 3(a)), which is the most elementary interaction in the neural network. The cross-correlograms in Fig. 3(b) show that clustered spontaneous activity was inhibited after an earlier clustered spontaneous activity. This indicates that a presynaptic neuron reduces the probability of firing in a postsynaptic neuron (Fig. 3(a)). These results show that dissociated cultures of cortical neurons can form functional synaptic connections on the polymerized MEA.
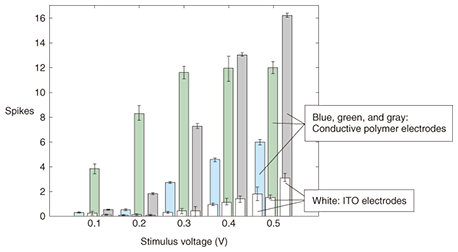
4. Neural stimulation through polymerized MEAWe then examined the stimulation properties of the polymerized MEA as an interface with a neural network of dissociated cortical neurons. The stimulation properties were investigated as a means of supplying the neural network with information. The stimulation efficiency at low stimulation voltages was evaluated and compared with that of ITO electrodes. We found that polymerized electrode stimulation evoked a much greater response from the network than stimulation with ITO electrodes. Stimulation trials were performed for three samples of conductive-polymer electrodes, as shown in Fig. 4. The minimum stimulus of 0.1 V for the polymer electrodes generated a weak response for two samples (blue and gray bars), while one sample (green bar) exhibited a clear response. For stimulus strengths above 0.1 V, all of the conductive-polymer electrodes evoked responses that were clearly stronger than that of the ITO electrodes (white). The activities evoked from two samples (blue and gray) increased steadily with stimulus strength but that of one sample (green) saturated after 0.3 V. For specific ranges, the activity was roughly proportional to the stimulus strength. The high stimulation efficiency achieved with polymer electrodes is due to the swollen nature of the polymer matrix in an aqueous environment, which provides high ionic conductivity and a large contact area between the electroactive material and the electrolyte.
Conductive-polymer electrodes can be used at low stimulating potentials for the efficient stimulation of neuronal tissue. They can also be tuned through a range of current densities, within a small voltage range, to allow for varying degrees of stimulation. The stimuli from conductive-polymer electrodes are more efficient than those from ITO electrodes. 5. Future prospects and challengesConductive polymers have recently attracted considerable attention as a material for neural network interfaces. Their conductivity enables the measurement of small signals in a neural network. The polymerized MEA can supply electrical stimulation to neural tissue using the same electrode as used for measurement while neural activity is being measured. Furthermore, the porosity of conductive polymers allows chemicals such as biogenic materials or drugs to be delivered directly to neural circuits. One of the problems we confront is the mechanical strength of the polymerized electrode. Delamination between the conductive polymer and the substrate can cause serious problems. However, Richardson-Burns et al. have successfully polymerized PEDOT around neurons [14], which indicates that conductive polymers can be smart materials that self-repair in living tissues. Therefore, we can use this polymerized MEA as an implantable communication device for a brain-machine interface or as an implantable medical device for neural prostheses. References
|
|||||||||||||||||||||||||||||||