|
|||||||||||||||||||
|
|
|||||||||||||||||||
|
Global Standardization Activities Vol. 8, No. 6, pp. 25–28, June 2010. https://doi.org/10.53829/ntr201006gls Development of Guidelines in Continua Health AllianceAbstractThis article reports on the activities and future outlook of the Continua Health Alliance (Continua), which is an open industry coalition established in June 2006 by founding members Intel, Philips, Panasonic, and other companies. Continua develops system-interconnectivity guidelines and certifies products with the goal of achieving an ecosystem of interoperable personal health management systems that will let people manage their personal health more effectively. More than 220 companies have become members of Continua (as of December 2009), and several products conforming to Continua design guidelines have been released since the second half of 2009.
1. IntroductionIn Japan, where specific health examination and specific health guidance became mandatory in fiscal year 2008, preventive healthcare is being increasingly promoted as a means of reducing healthcare expenses in the future. From a global perspective, about 1 billion people are overweight and about 860 million people have chronic diseases. Healthcare expenses incurred for chronic diseases make up a very high proportion, namely, 75–85%, of total healthcare expenses. In addition, the number of people aged 60 or older has reached about 600 million worldwide, and it is accepted that people in this age group will need health management on an ongoing basis from preventive healthcare to actual medical care. Against this background, the Continua Health Alliance (hereinafter, Continua) [1], is involved in diverse activities including studying use cases, creating guidelines for system interconnectivity, performing connection tests using actual equipment and devices, and managing a logo certification program (Fig. 1) for the three fields of (1) health and wellness, (2) disease management, and (3) aging independently.
2. Organization and Working Group activitiesAs summarized in Table 1, Continua consists of seven Working Groups (WGs). The Use Case WG selects and analyzes use cases in detail, and the Technical WG defines requirements for each use case, selects appropriate connection standards, and creates guidelines. The Test & Certification WG develops test methods and a certification program and manages a list of certified products. There is also a Marketing WG that promotes events and public relations activities. There are over 1900 members participating in the various working groups.
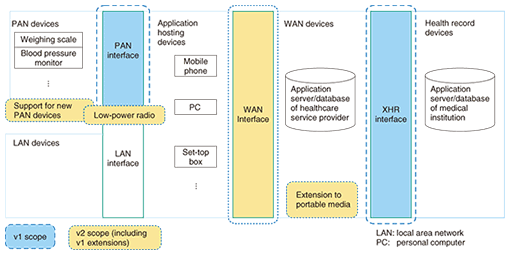
3. System architecture and scope of guidelinesIn its design guidelines, Continua prescribes the devices and interfaces shown in Fig. 2 to handle a range of processes from the collection of vital signs to the storage of that information on application servers or databases of healthcare institutions. Version 1 (v1) of the Continua Design Guidelines, released in February 2009, deals mostly with the following interfaces shown in Fig. 2.
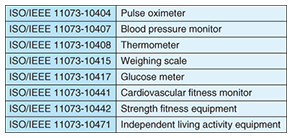
(1) Personal area network (PAN) interface between PAN devices and application hosting devices (2) Data format and electronic/personal health record (XHR) interface between wide area network (WAN) devices and health record devices Specifically, Design Guidelines v1 prescribes the eight PAN devices listed in Table 2. These devices connect to application hosting devices like a personal computer or mobile phone to transfer collected data. The USB Personal Healthcare Device Class Specification and the Bluetooth Health Device Profile Specification were chosen to establish wired and wireless connections, respectively, between these devices (USB: universal serial bus). For actual data transfers, the Guidelines prescribe the IEEE11073-20601 communication protocol for optimizing data exchange and specify protocols and data formats based on the various device specifications listed in the table [2], [3].
At present, studies are being performed to support new PAN devices, low-power radio, a WAN interface, etc. toward the creation of Design Guidelines Version 2 (v2). Several companies have already announced Continua-certified products based on Design Guidelines v1. In Japan, the first ones announced were a blood pressure monitor and weighing scale, and Continua-certified products from manufacturers of vital-signs sensors are now being announced one after the other [4]–[6]. 4. Continua activities in JapanIn Japan, the Japan Regional Committee for Continua (Continua-J) [7] centered on Intel Japan held press events in February 2009 and February 2010 (Photo 1) [8] in addition to holding meetings. NTT participates in Continua-J and is working to prescribe operations that will facilitate the efficient introduction of Continua-compliant equipment and devices into actual services.
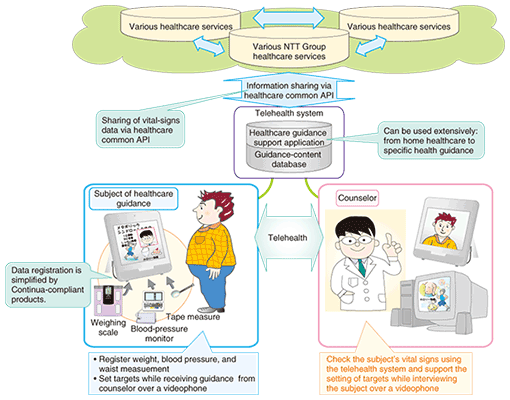
5. Current R&D at NTT and future outlookNTT is promoting the development of telehealth solutions that will enable vital signs to be easily registered using Continua-compliant products and health guidance to be given remotely. It is also formulating a common interface (healthcare common API (API: application program interface)) to enable healthcare data to be shared among healthcare service providers (Fig. 3). The results achieved by these research and development efforts will be made available to the NTT Group toward the provision of new healthcare services.
Looking forward, NTT aims to develop technologies for upgrading the healthcare common API and supporting new PAN devices while following developments in Continua Design Guidelines v2, especially with respect to the WAN interface. In doing so, it hopes to contribute to a revitalized NTT Group and Japanese healthcare market. 6. ConclusionWith the aim of overcoming healthcare-related problems facing the entire world, Continua and its participating members have developed Design Guidelines v1 and v1-compliant products toward an ecosystem of interoperable personal health management systems using information and communications technology. The Continua coalition plans to update its Design Guidelines on an ongoing basis and continue to develop products toward the spread of personal healthcare and the creation of a healthy society. References
|
|||||||||||||||||||