|
|||||||||||
|
|
|||||||||||
|
Practical Field Information about Telecommunication Technologies Vol. 8, No. 12, pp. 34–39, Dec. 2010. https://doi.org/10.53829/ntr201012pf1 Coatings for Protecting Telecommunication Steel TowersAbstractThis article describes coatings for protecting telecommunication steel towers. It is the second in a new bimonthly series on the theme of practical field information about telecommunication technologies. This month°«s contribution is from the Materials Engineering Group, Technical Assistance and Support Center, Maintenance and Service Operations Department, Network Business Headquarters.
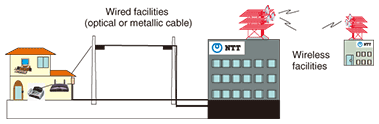
1. IntroductionNTT provides telecommunication services by both wired facilities such as optical and metallic cables and wireless facilities for mobile communications (Fig. 1). Major NTT buildings invariably have either gray (Fig. 2) or red-and-white (Fig. 3) steel towers on their roofs, which are essential to wireless communications. These towers are so familiar that many people use them as landmarks when giving directions and finding their way around. This article describes coatings for protecting steel towers from corrosion while referring to a coatings inspection manual that was revised last year. It also touches upon bridges, whose steel materials are likewise protected by coatings, and discusses current issues for the use of paints.
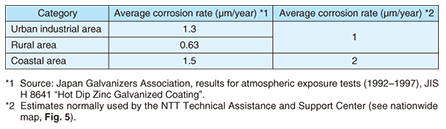
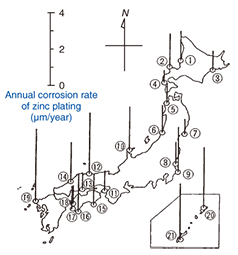
2. Outline of steel tower coatingsSteel towers used for telecommunications consist of steel materials treated by hot-dip galvanization (zinc plating). Specifications for main bridge components call for HDZ55 plating with 550 g of zinc per 1 m2 and an average film thickness of about 80 ¶Őm, while those for hand rails and gratings call for 350 g of zinc per 1 m2. It was originally accepted that zinc plating would halt the progress of corrosion through the formation of white rust (mainly zinc hydroxide), which provides protection. However, in environments where corrosion can be severe such as islands and coastal areas and hot-spring areas, there are many situations in which corrosion can progress, as listed in Table 1, leading to the formation of red rust (iron oxide) (Fig. 4). Moreover, if the corrosion of steel together with wastage is allowed to continue, the main columns or other components of a steel tower may have to be replaced.
2.1 Reasons for coatingsTelecommunication steel towers are coated for the following reasons, including coating repair to prevent corrosion-related failures. (1) To apply daytime markings indicating obstacles, as stipulated by Japan°«s Civil Aeronautics Act*1 (seven alternating red and white bands required) (2) To observe directives from the Ministry of the Environment (with respect to national parks, natural parks, etc.) (3) To repair zinc-plated steel towers 2.2 Colors of coatingsThe colors of coatings for steel towers are listed in Table 2.
2.3 Roles of coatingsThe main purpose of a coating is to prevent corrosion of steel materials treated by hot-dip galvanization. The standard film thickness of a coating is at least 125 μm in a general environment and at least 250 μm in a highly corrosive environment such as an area susceptible to attack by chloride. The features and purpose of each layer of applied coating are summarized in Table 3.
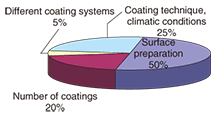
2.4 Past steel-tower coating failuresIn recent years, some steel towers located in ordinary areas that were coated according to specifications in the current inspection manual (second edition) have had to be recoated because of peeling (Fig. 6) within four or five years of painting. A breakdown of past coating failure causes is shown in Fig. 7. They reveal the need to revise those specifications.
3. Revision of inspection manualThe following is an extract from the “Inspection Manual for Steel Towers, Antennas, and other Outdoor Telecommunication Facilities” (Third Edition; revised June 30, 2009): “To make the work of maintaining and managing steel towers more appropriate, efficient, and smoother, the inspection method and repair criteria in the current manual have been revised and surface preparation, numbers of coatings, and other processes have been standardized and the use of weak-solvent paints specified to extend the lifetimes of steel-tower coatings.” The main changes in the 2009 revision are summarized below. (1) Changes in inspection method In the past, the discovery of peeling, cracking, transparency (Fig. 8), or other faults was followed by a detailed inspection: the area taken up by each fault was calculated and organized by fault type and location, and the ratio of affected areas to total area was computed as a criterion for repainting. This complicated method suffered from variance in evaluation and required skilled inspectors. In the revised inspection manual, the criterion for repainting (Table 4) is the quantitative degree of deterioration as determined by a visual inspection and cross-cut test (Fig. 9), two relatively simple inspection items.
Visual inspection: Checks deterioration of coating on the basis of its external appearance Cross-cut test: Examines adhesion of paint film to steel material to determine the degree of deterioration when no anomalies can be visually observed (2) Changes in surface preparation grades For existing steel-tower coatings, the standard for surface preparation has been Grade 3, in which the old paint film is left and new paint is applied on top of the old paint. However, old paint films are often more than 30 years old, and deterioration such as thinning caused by ultraviolet rays, wind, and rain can cause the adhesion between the old film and the steel to deteriorate. There are consequently many examples of paint film peeling from the interface with the steel material. We have therefore changed (standardized) the surface preparation to Grade 2, which stipulates complete removal of the old paint film using a power tool (see Table 5). This specification results in good adhesion and thereby prevents peeling due to deteriorated paint films.
(3) Process standardization Environment: maximum/minimum air temperatures of 40°C/5°C and humidity below 85%. Coating method: brush-based as standard. Paints from the same manufacturer shall be used in principle. (4) Paint recommendations With the aim of preserving the global environment, NTT recommends the use of weak-solvent paints from various manufacturers that have passed standard tests. These paints should be applied according to established values for film thickness and number of coats. The drying time for each coat should also be observed. 4. Coating specifications for bridgesLike steel towers, bridges involve the coating of large-scale steel structures (Figs. 10 and 11). Below, we present examples of coating specifications for steel bridges from NTT (in relation to bridge-suspended facilities) and the Japan Road Association, a public-interest corporation under the Ministry of Land, Infrastructure, Transport and Tourism.
NTT-recommended coating specifications are listed in Table 6. If at all possible, surface preparation shall be performed by Grade 1 blast cleaning. However, if blasting equipment cannot be set up, Grade 2 cleaning, in which the old paint film is completely removed, can be used.
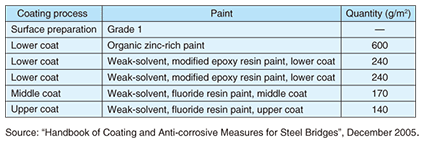
An example of repaint-coating specifications of the Japan Road Association is given in Table 7.
5. Current issues for paintsPaints for coating steel towers often used to contain volatile organic compounds (VOCs)*2 such as toluene and xylene as solvents. Since solvents such as these can be toxic to humans as well as a heavy burden on the environment, measures are being taken around the world to reduce their use. The recommended paints in the newly revised inspection manual reduce these solvents by about 50% compared with 2000 levels. Regulations on VOC emissions have already been introduced in the paint and coating industry, and the Ministry of the Environment and local governments like the Tokyo Metropolitan Government are implementing similar measures. Some examples of these efforts are given below. (1) Tokyo Metropolitan Government, Bureau of EnvironmentThe Tokyo Metropolitan Government aims to achieve the following (according to its website).
(2) Japan Paint Manufacturers AssociationThe Japan Paint Manufacturers Association (a public-interest corporation under the Ministry of Land, Infrastructure, Transport and Tourism) published “Guidelines for Control of Volatile Organic Compound (VOC) Emissions” in May 2004 calling for a 50% reduction in emissions by 2010 compared with 2000 levels. (3) Japan Road AssociationThe Japan Road Association (a public-interest corporation under the Ministry of Land, Infrastructure, Transport and Tourism) published the “Handbook of Coating and Anti-corrosive Measures for Steel Bridges” in December 2005 calling for coating specifications that excel in durability to reduce life cycle costs. It recommends weak-solvent paints and proposes aqueous solvents. (4) Other related laws and regulationsAn Ordinance of the Ministry of the Environment, “Control of VOC Emissions (amendment to the Air Pollution Control Law)”, was enacted on May 27, 2005 and implemented on April 1, 2006 [1]. The Law Concerning the Promotion of Eco-Friendly Goods and Services by the State and Other Entities (Green Purchasing Law) was enacted on May 31, 2000 and implemented on April 1, 2001 [2].
6. ConclusionThis article described the use of coatings to protect telecommunication facilities and presented specific examples of coatings. The members of the NTT Group plan to work together to contribute to the preservation of the global environment with regard to paints and coatings. References
|
|||||||||||