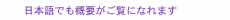|
|||||||||||||||
|
|
|||||||||||||||
|
Feature Articles: Environment and Energy Technologies Toward a Green Society Vol. 9, No. 2, pp. 37–43, Feb. 2011. https://doi.org/10.53829/ntr201102fa6 Anti-corrosion Technologies for Telecommunications Structures and EquipmentAbstractIn this article, we introduce research on anti-corrosion technologies with a focus on outdoor telecommunications facilities and equipment. This research is proceeding in two ways: by understanding individual corrosion mechanisms for metallic materials at the microscopic level and by rapidly visualizing corrosion risks from the macroscopic viewpoint. We explain these two approaches and present the latest research results.
1. Installation environment for telecommunications structures and equipmentToday’s information and communications technology (ICT) is supported by many types of structures and equipment. A typical scenario is shown in Fig. 1 [1]. These structures and pieces of equipment include many outdoor transmission paths such as optical fiber cables for conveying information from a switching center to the customer’s home and equipment for physically supporting those paths such as utility poles.
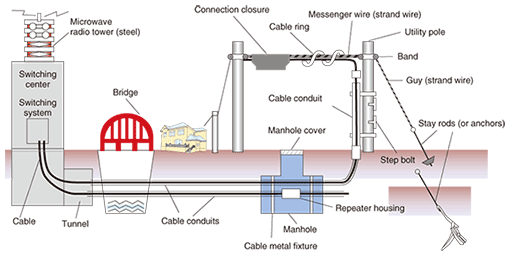
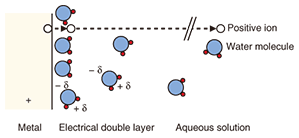
Since these facilities are installed everywhere in Japan to provide universal service, they are placed in a wide variety of environments including mountainous regions, coastal areas, hot-spring areas, and regions with heavy snowfall. They are exposed to the natural environment, which means that they can be affected by the annual and daily range of temperature, annual rainfall, hours of daylight, and other natural phenomena. Since we cannot control the natural environment, we must find means of preventing corrosion and other types of deterioration in facilities caused by atmospheric factors. 2. Corrosion in telecommunications structures and equipmentTelecommunication structures and equipment are typically constructed from metals (especially steel) and plastics. These materials have very different characteristics, but both of them will deteriorate over the long term when exposed to the natural environment. Steel can give rise to corrosion products (rust) if exposed to water or salt, and plastics can suffer from blanching and degraded mechanical characteristics since ultraviolet rays from the sun can break down molecular chains and reduce the molecular mass. Moreover, guy wires swinging in the wind can suffer from metal fatigue, and cables can be bent by the weight of accumulated snow, leading to a deterioration phenomenon called galloping. While many problems can arise from the mutual interaction between the natural environment and the material components of structures and equipment, the major one is metal corrosion. Corrosion has been a problem for a long time, and research into countermeasures at NTT has been ongoing since the establishment of the Technical Assistance Section in the Electrical Communication Laboratories of Nippon Telegraph and Telephone Public Corporation (the forerunner to NTT) in 1963 [2]. However, as new telecommunications technologies and facilities come to be developed and structures and equipment come to be deployed in even more severe environments, the need for new anti-corrosion measures is growing. At the same time, concerns have been rising in recent years about corrosion in aging structures and equipment that was installed in great quantities during Japan’s high-growth era. In short, we have entered an era that demands measures for protecting telecommunications facilities from corrosion and prolonging their life. 3. Corrosion mechanism in steel strand wiresMost steel used in outdoor structures is treated with anti-corrosion measures. Typically, steel is protected from corrosion by zinc coating. The ability of zinc coating to counteract corrosion in steel is explained by the following two functions (Fig. 2).
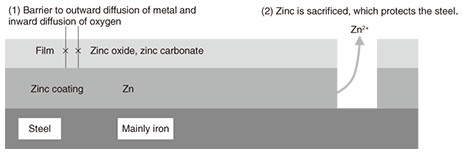
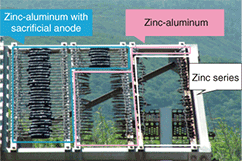
(1) When zinc corrodes by itself, it forms a film that delays further corrosion (by hindering the outward diffusion of metal and inward diffusion of oxygen). (2) The electrical potential of zinc corrosion in water is lower than that of iron, so even if the zinc coating should be damaged and expose the iron, the zinc will corrode first and thereby protect the steel. In other words, steel, if covered by a film of water generated, for example, by condensation, will turn into iron ions and bond with oxygen in the atmosphere, thereby becoming an iron oxide, which has different characteristics to steel. The zinc-based anti-corrosion method works to defeat this process by preventing such diffusion and electrochemical reactions. In particular, the second of the above two effects, whereby steel is protected even if the zinc coating is slightly damaged, makes zinc coating more effective than paint. On the other hand, the fact that zinc coating is effective in counteracting corrosion does not mean to say that it can completely prevent corrosion from occurring. In coastal areas, for example, sea-salt accelerates corrosion, which creates a need for coatings composed of materials such as aluminum or zinc-aluminum alloys that have greater anti-corrosion properties. In this regard, our research group conducted a study comparing corrosion among steel strand wires with various types of coatings after exposure for more than 20 years near the coast on an island group near Tokyo [3]. As shown in Fig. 3, steel strand wires with zinc plating lost their plating during the exposure period resulting in corrosion of the underlying steel, while the steel strand wires with aluminum cladding were generally sound except for sections with crevices. On the other hand, anti-corrosion processing involving the application of aluminum-series alloy tape protected those sections with crevices.
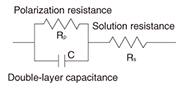
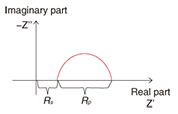
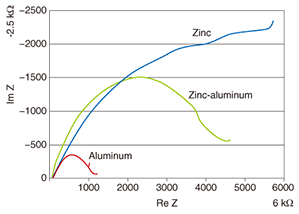
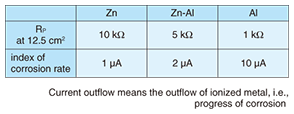
On the basis of the above results, we researched the corrosion mechanism with a focus on how corrosion begins in crevices in steel strand wires in the case of different types of coatings [4]. Corrosion in iron has already been explained in terms of electrochemical reactions (battery cell reaction) via ions, and with this in mind, we arranged two reed-shaped samples in parallel to form a crevice and performed an experiment to quantitatively determine how an electrical circuit can form and how corrosion can proceed using the crevice spacing and humidity as conditions (Fig. 4). The model that we used here for the metal/water-film interface during the corrosion process is shown in Figs. 5 and 6. If such an electrical circuit is formed, the impedance components between the samples can be described from circuit theory as a real part and an imaginary part that draw out a semicircle, as shown in Fig. 7. In other words, in a system in which AC voltage at a certain frequency is applied to a corroding system and the impedance is measured, the difficulty of corrosion (Rp) can be determined graphically. On the basis of this idea, we left a sample with a crevice spacing of 1 ¦Ìm in an environment with relative humidity of 98%, and noted that, while no electrical circuit had formed after six hours, one had formed after 25 hours with a portion of an arc appearing. We performed the same experiment for zinc, aluminum, and a zinc-aluminum alloy (55% aluminum) and obtained the arcs shown in Fig. 8. Examining these results, we can see that those for zinc have the largest radius with only part of the arc apparent. Those for the zinc-aluminum alloy, meanwhile, exhibit nearly the entire semicircle, and those for aluminum have the smallest radius. Thus, if we now determine the characteristics of the circuit elements from these results and measure the corrosion rate, we can tabulate our results (Table 1). Since current outflow is determined by the outflow of ionized metal, i.e., by the progress of corrosion, it can be seen that the corrosion of aluminum in crevices can easily progress.
To put it another way, two things have become qualitatively and quantitatively understood: (1) a battery cell can form and metal can begin to corrode depending on the size of the crevice even for a level of humidity that has not reached the dew point and (2) the progress of corrosion in crevices differs from one type of metal to another. It is therefore essential to give special attention to crevices when protecting metal structures with a coating of aluminum or other metal; this is more important than when protecting zinc-plated structures, even in sub-dew-point conditions. A technique like the one described above lets one research where on structures or equipment metal corrosion can easily begin and under what conditions the risk of corrosion can increase. 4. Regional differences in corrosionJapan features a variety of natural environmental conditions. The problem of humidity, for example, differs between Hokkaido and Okinawa. Even though the corrosion of specific structures and equipment is affected by the moisture conditions and the crevices in question, it must be considered that the average corrosion rate will be analyzed by regional corrosion conditions. The fundamental mechanism of metal corrosion, as explained above, is the transformation of metal to ions and the flow of current. However, given that salt dissolved in water creates an electrolyte solution with low resistance and that salt being deliquescent can instigate the formation of water films, it should be kept in mind that structures and equipment in locations where salt can easily adhere and be absorbed, such as in coastal regions, are especially susceptible to severe corrosion (saline damage). Accordingly, to investigate regional differences in assumed corrosion environments, we constructed a prototype system for estimating corrosion rates throughout Japan with special attention paid to saline damage. The model for this system is based on a previously disclosed model researched by NTT [5]. The system is summarized in Fig. 9. Using actual measurement values from 21 observation regions throughout Japan, this system aims to visualize risk by estimating values (1)–(4) described below at any point on the Japanese map.
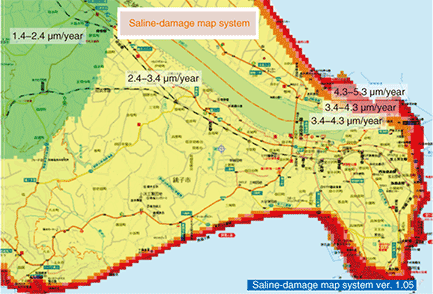
(1) Amount of sea-salt aerosol: Indicates the quantity of sea-salt particles adhering at a certain point per unit area per day (unit: mg/cm2/day). Assuming that marine aerosols generated by the ocean can be transported by the wind, the quantity of sea-salt particles can be directly calculated from wind direction and speed for the day in question by incorporating a damping expression for the concentration of particles swept inland from the coast by using an exponential function. However, as meteorological data cannot be obtained for all points, the system extracts data from the nearest observation points in Japan’s Automated Meteorological Data Acquisition System (AMEDAS) and interpolates data (using the same interpolation method as for calculating values (2)–(4) below). (2) Corrosion rate: Indicates the wastage per unit time of metallic material covering the surface of the target structure (unit: ¦Ìm/year). The corrosion rate is computed from the amount of sea-salt aerosol adhering to the metallic surface, the metallic material used, temperature obtained from AMEDAS data, and moisture-related factors (number of days with condensation and amount of rainfall). At present, calculations can be performed for iron and zinc (the main component of plating). (3) Amount of corrosion: Indicates the amount of wastage at a certain point in time (unit: ¦Ìm). This is the integral of corrosion rate for the period in question, but in this model, we also consider that the corrosion rate decreases as the amount of corrosion increases (time to the -0.3 power) [6] (based on an empirical formula for quantifying function (2) in Fig. 2). (4) Remaining life: Indicates the time required for the current plating thickness to reach either zero or a certain specified value (unit: months). This value is determined by extrapolating the amount of corrosion and the corrosion rate at the time of calculation. The initial value of film thickness and the year that the structure was built or installed must be given. These values and the quality of the material used are available from the facility database. A screenshot of simulation results obtained using this system is shown in Fig. 10. This system supports interactive operation using a mouse: selecting a point or region on the map superimposes simulation results on the screen. Color is used to display corrosion results to enable the user to readily understand how corrosion spreads near the seashore. This system therefore enables the extent of saline damage for certain regions (shown by different colors) to be estimated from publically available meteorological data and geographical information. As such, we can expect it to be used as a reference source when upgrading or triage-operating equipment susceptible to saline damage. Moreover, since the system also provides the distribution of corrosion rate, it lets us enhance facility repairs more efficiently.
5. Concluding remarksThis article introduced research on anti-corrosion technologies with a focus on outdoor telecommunications facilities. We plan to increase the operation speed of our prototype system, test its validity for actual degradation phenomena, and develop it into a practical system while considering various usage scenarios. References
|
|||||||||||||||