|
|||||||||||||||
|
|
|||||||||||||||
|
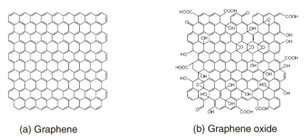
Feature Articles: Front-line Research on Graphene Vol. 11, No. 8, pp. 35–39, Aug. 2013. https://doi.org/10.53829/ntr201308fa7 Biosensing on a Graphene Oxide SurfaceAbstractThis article describes our recent research on a selective protein detection system built on a graphene oxide (GO) surface. Our original idea is to fix pieces of GO on a solid support. This enables direct observation of chemical reactions occurring on the GO surface and on-chip device fabrication. The basic principle can be applied to other proteins as well as other biological molecules simply by changing the molecules to be modified, which makes GO a versatile platform for integrated biosensing devices. 1. Introduction to graphene oxideGraphene oxide (GO) has a sheet-like structure similar to graphene, but its preparation method and electronic properties are very different from those of graphene. Let us take a brief look at the similarities and differences between GO and graphene. The schematic chemical structure of graphene and GO are shown in Fig. 1. Graphene consists solely of benzene rings made of carbon (C) atoms with C=C double bonds (sp2 bonds). In GO, many of the double bonds in graphene are scissored and linked to oxygen (O) to form C-O bonds, which yields C-C single bonds (sp3 bonds). Mobile π electrons that dominate the conductivity in graphene are lost at the oxidized bonds. Thus, GO becomes an insulator. Many articles in this special issue describe research concerning the conductive nature of graphene, and this is a big difference between the two materials.
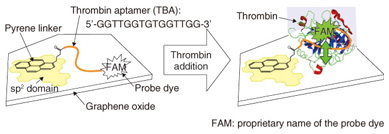
The preparation of GO is totally different from that of graphene. We can chemically synthesize GO by oxidizing graphite in strong acid conditions [1]. This yields a large quantity of GO in glassware. In contrast to graphene, GO is well dispersed in water. This is because of the many hydroxyl (-OH) groups existing on the GO surface. The synthesized GO has a sheet-like structure about 1 nm thick and with 1–100 µm edges, which was determined by atomic force microscopy (AFM) [2], [3]. GO is a very large material compared with molecule-scale materials. It is surprising that such large pieces of GO can float and are not precipitated in water. Note that GO does not dissolve in water: it forms a colloid solution. Thus, GO is precipitated when we centrifuge the solution. 2. Mechanism of biomolecule detectionGO is known to behave as a very efficient fluorescence quenching material [4]. When a fluorescent molecule such as a dye is located in the vicinity of the GO surface, the fluorescence from the dye is not observed. As the distance between the dye and the GO surface increases, the quenching efficiency drastically decreases, and the dye fluorescence becomes observable. This is an important feature of the biosensing architecture presented in this article. We fabricated the protein detection system on the surface of GO that is fixed on a solid support through chemical modification [5]. We first explain the fabrication method and the detection mechanism. GO that is fixed on a solid support is readily prepared by spin-coating an aqueous dispersion of GO on a hydrophilic surface such as a silicon (Si) wafer with silicon dioxide (SiO2) (SiO2/Si) and quartz. The density of GO pieces can be controlled by the concentration of the dispersion and the spin-coating conditions. For the experiments described here, we used relatively large pieces of GO fixed on SiO2/Si at low density in order to access a single piece of the GO. The function for biological molecule detection can be built on the GO surface through chemical modification (Fig. 2). There are many sp2 domains, which remain as graphene-like structures, in GO. Pyrene, a molecule made of four benzene rings, is known to have a strong affinity to graphene surfaces. We thus prepared pyrene with a reactive site (a succinimide group) and adsorbed the pyrene molecule to the sp2 domains in GO.
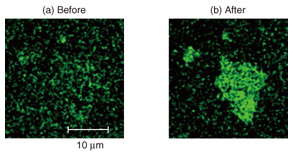
Deoxyribonucleic acid (DNA) was linked to the reactive site in the next step. DNA is a polymer composed of four bases that forms a double helix structure with its complementary DNA. The DNA we used here is called an aptamer, which is single-stranded DNA with a determined base sequence. The aptamer can bind with a specific protein molecule to form a complex structure. Biosensors that use aptamers are often referred to as aptasensors. We used a thrombin aptamer, which forms a complex with thrombin, a protein important in blood clotting. Thrombin is produced in our body after an injury. It facilitates clotting in order to prevent the loss of blood. It is not ordinarily present in the blood; if it were, it might induce an infarct in a blood vessel. An infarct is an area of tissue that undergoes necrosis as a result of an obstruction in the blood supply. The base sequence of the thrombin aptamer is shown in Fig. 2. One of the aptamer termini has an NH2 group that forms a chemical bond with a pyrene linker. The other is bonded to a dye used for the detection. The intended base sequences with modified termini can be easily synthesized by modern bioengineering. We can therefore order and purchase the designed DNA molecule. The single-stranded DNA has a great affinity to the GO surface and is adsorbed on the surface in both atmospheric and solution conditions. Thus, the dye molecule is located in the vicinity of the GO surface, and its fluorescence is not observed because of the efficient quenching by GO. Since the biomolecule usually functions in solution conditions, let us consider the case where we add thrombin into a molecular system in water. As shown in Fig. 2 (right), the aptamer recognizes thrombin and forms a three-dimensional complex structure. This forces the dye at the terminus to be separated from the GO surface. Then the dye fluorescence that was quenched at the initial stage is recovered. Although the change in distance is very small, the recovery sensitively responds to the difference. By observing the recovered fluorescence, we can detect the protein, in this case thrombin. 3. Direct observations of protein detection on GO surfaceWe show the experimental results of thrombin detection in this section. The fluorescence microscope images of a GO aptasensor before and after thrombin addition are shown in Fig. 3. The image was dark before the addition; it fluoresces green after the addition. This is attributed to the fluorescence from the dye at the aptamer terminus. We demonstrate the protein detection using our GO aptasensor in this manner. Time lapse observations of the green fluorescence revealed that the fluorescence intensities become about four times more than the background fluorescence. As we can imagine from the fluorescence images, the increase is significant enough to detect the specific proteins.
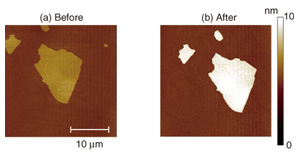
A detection system similar to the one we constructed on GO has been reported by other research groups, but they demonstrated the detection using an aqueous dispersion of GO [6]. The means of biosensing using a GO aptasensor fixed on a solid support is NTT’s original idea [5]. The fixation enables us to perform several interesting experiments that have not been possible with the dispersion system. The images shown in Fig. 3 were taken by a confocal microscope that detects fluorescence only at the focused plain. Therefore, the green fluorescence in Fig. 3 originated from the complex formation between thrombin and the thrombin aptamer on the GO surface. However, it is impossible to determine how much thrombin adsorbs on the surface, and how densely, only from the fluorescence intensities. To estimate them, we observed the GO surface using AFM, which cannot be applied to GO in aqueous dispersion. By observing the same piece of GO using a confocal fluorescence microscope and AFM, we can make a reliable determination. The AFM topographies before and after thrombin detection are shown in Fig. 4. The shape of the piece corresponds to that of the green fluorescence in Fig. 3, which is good proof that we are observing the same piece of GO. The average thickness of GO is 1.7 nm before and 3.6 nm after the thrombin detection. This obvious increase in thickness is due to the thrombin adsorption. The thrombin is about 5 nm in diameter in solution conditions. The increase in the thickness is smaller than the diameter, which suggests that the thrombin does not fully cover the GO surface. In addition, the AFM topographies were taken in dry atmospheric conditions, which may cause transformation and shrinkage of the thrombin. Another important feature in Fig. 4 is that no thrombin was observed on the hydrophilic SiO2 surface. Although proteins like thrombin often adsorb on many kinds of surfaces, it is suppressed in our system. We would therefore not have been able to detect the selectivity of thrombin adsorption without the AFM observations.
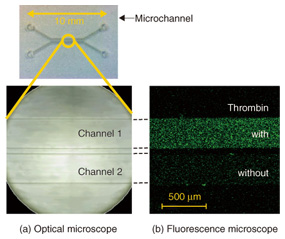
4. On-chip device applicationThe unique feature of our system is that the GO aptasensor is fixed on a solid support. This enables us to introduce the microchannel device on the GO aptasensor and to extend the technology to on-chip device applications. The prototype device, the GO aptasensor equipped with two microchannels, is shown in Fig. 5 [7].
Practical sensor applications always require high sensitivity. We expect that higher sensitivity is achieved with this device by using substrates covered densely with GO, rather than the isolated GO as in Fig. 3. We prepared such substrates by spin-coating fine pieces of GO onto them. The fine GO had edges less than 1 µm, which was obtained by irradiating the ultrasound beam to the aqueous dispersion of the original GO. The appropriate conditions will yield substrates with more than 70% surface coverage by GO. The substrate was modified with the aptamer and overlaid with the dual microchannel on the top. The fluorescence microscope image of the microchannels filled with thrombin solution (channel 1) and water (channel 2) is shown in Fig. 5. It is clear that the whole area is green fluorescent in channel 1. This originates from the dye tethered on the GO surface fixed on a solid support. By contrast, the green fluorescence is at a background level in channel 2, which has no thrombin in the solution. One of the advantages of the microchannel configurations is that we can examine the characteristics of the GO aptasensor easily and accurately. This is because we can compare the response of the aptasensor to several different samples at the same time, while maintaining the other identical experimental conditions. This is a powerful technique for optimizing the molecular structure in order to achieve higher sensitivity and for estimating the selectivity of proteins. 5. Future perspectivesIn this article, we presented our protein detection system built on a GO surface and its use in detecting thrombin. We combined materials science research conducted in NTT Basic Research Laboratories and microfluidics technology developed in NTT Microsystem Integration Laboratories to develop an original idea that has many advantages and potential applications in various areas of research. In future, the protein detection system can be applied to a variety of target molecules by choosing the corresponding aptamers. We have already demonstrated the detection of PSA (prostate specific antigen), a protein known as a cancer marker. The detection sensitivity can be increased by designing the molecular structure for GO surface modification. We have also demonstrated a significant increase in the thrombin detection sensitivity by adding a DNA spacer. The accumulation and combination of individual technologies is expected to lead to an integrated on-chip GO aptasensor that enables us to simultaneously detect a wide variety of biological molecules with a small quantity of samples solution. References
|
|||||||||||||||