|
|||||||||||
|
|
|||||||||||
|
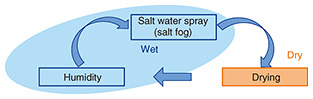
Feature Articles: Research and Development of Life-cycle Maintenance of Telecommunication Infrastructure Vol. 16, No. 1, pp. 26–31, Jan. 2018. https://doi.org/10.53829/ntr201801fa3 Accelerated Corrosion Test for Evaluating the Corrosion Resistance of CoatingsAbstractWe developed a new accelerated corrosion test for evaluating the corrosion resistance of coatings. The new test was designed to approximate the water absorption and desorption behavior of anti-corrosion coatings in actual outdoor environments. This test makes it possible to well approximate atmospheric corrosion in seafront areas and to accelerate the corrosion of steel and zinc by around 1.4 times compared with the conventional method. This test will make it possible to efficiently and accurately select coatings in a shorter test time. Consequently, the maintenance cost of steel structures can be reduced by selecting the appropriate coatings. Keywords: coating, accelerated corrosion test, cyclic corrosion test 1. IntroductionThe NTT Group has a huge number of outdoor telecommunication facilities, with many composed of metallic materials or painted metallic materials. Whether outdoor steel structures such as wireless towers on NTT buildings should be painted and repainted is determined on the basis of inspection results. The NTT Group has many outdoor steel structures, so the cost of painting is huge. Furthermore, in the near future, the number of facilities that need new paint is expected to increase, which will drastically increase maintenance costs. Therefore, highly reliable and long-lasting coating materials with high resistance to corrosion must be selected in order to reduce the maintenance costs of outdoor steel structures. The selection process involves conducting various accelerated tests to evaluate the performance of coatings. Coatings that surpass the criteria are subsequently adopted. 2. Accelerated corrosion tests for evaluating coatingsThe various accelerated tests for coatings include accelerated corrosion tests for evaluating resistance to atmospheric corrosion in seafront areas. The representative accelerated corrosion tests are the salt spray test (SST), which uses a continuous salt water spray (salt fog), and the cyclic corrosion test (CCT), which repeats salt fog, drying, and humidity steps (Fig. 1).
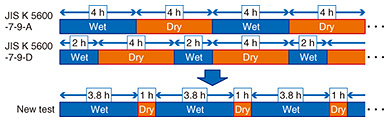
In the past, the SST was widely used as a basic accelerated corrosion test. However, since corrosion behaviors differ considerably between the SST and outdoor environments, the performance of coatings evaluated using SST does not necessarily match the performance of coatings in those environments. Therefore, the CCT has become popular over the past 20–30 years. The CCT is certainly an excellent accelerated corrosion test that has few problems, but it requires a very long test time. With CCT, the corrosion resistance and lifetime of the coatings have improved year to year, but at the cost of longer test times to judge the comparative merits and demerits of the improved coatings. Therefore, there is an increasing need to shorten the test time. In this article, we introduce a new CCT called the NTT Cyclic Corrosion Test (CCT-N) that can accelerate corrosion compared to the conventional SST and CCT and reproduce the corrosion behavior of outdoor exposure. 3. Conventional CCT and new hypothesisDespite the increasing demand for shortening the test time, a method to further accelerate corrosion in the CCT has not been studied in recent years. In the prevailing test conditions in the existing CCT (JIS* K 5600-7-9 methods A and D) in Japan, drying times account for over half of the total test time (Fig. 2). Since corrosion does not proceed after a sample has completely dried, the average corrosion rate (the speed at which a metal deteriorates in a specific environment) could be improved and the test time shortened by reducing the drying time. However, when the CCT was developed, it was considered that a low wet-time ratio was absolutely essential for approximating the actual environmental corrosion. The wet time ratio is defined as the sum of the times for the salt fog and humidity steps divided by the total testing time. Wet time ratios of 50% or less have been recommended up to the present [1]. In addition, coating standards in Japan stipulate that many kinds of coatings should be evaluated using JIS K 5600-7-9 method A or D; therefore, there was not much motivation to modify the test conditions. For these reasons, much research in recent years has been aimed at approximating atmospheric corrosion in seafront areas rather than improving the corrosion rate and shortening the test time.
In contrast, we consider that a continuous wetting time, not the wet time ratio, is of major importance for achieving corrosion behavior similar to that in outdoor exposure. Normally, absorbed water in the coatings takes some time to reach the coating/steel substrate interface. Consequently, the behavioral variation in water quantities at the interface will be very different between short dry-wet intervals and long dry-wet ones even if the wet time ratios of the CCTs have the same value. Thus, we focused on the behavior of water absorption and desorption in the coating film. Water and oxygen are necessary for corrosion to progress. When the coating film is dry, only oxygen is present in it. After wetting, water is absorbed into the coating film and reaches the interface between the coating/steel substrate, and the steel is corroded by water and oxygen. In contrast, if the coating has been wet for a long time, oxygen will run short at the coating/steel interface. In actual outdoor environments, this wetting and drying is repeated. In an accelerated corrosion test, in order to accurately simulate coating corrosion in actual outdoor environments, it is desirable to set the test conditions of the CCT so that the water absorption of the coating film is not excessive or insufficient, as in the actual outdoor environments. In addition, a wet time ratio of more than 50% of the test time is not considered to be a problem. A survey of the CCT literature revealed that authors consider a continuous wetting time of four hours or less to be appropriate in CCT. From this consideration, we decided to increase the wet time ratio and set the continuous wetting time at four hours or less in our new CCT.
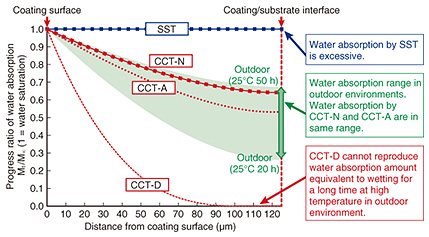
4. Test conditions of CCT-NIn this section, we explain the key elements of the CCT-N test conditions. 4.1 Temperature dependenceIn accelerated corrosion tests, corrosion is generally accelerated at temperatures higher than those in the actual outdoor environments. The temperature in the salt fog, drying, and humidity steps differs depending on the CCT. The water absorption and desorption rates of the coating films proceed faster as the temperature increases. For this reason, we considered that it is insufficient to simply set the continuous wetting time at four hours or less and that the wetting (salt fog and humidity) time and drying time should be considered with this temperature dependence taken into account. Therefore, we investigated the temperature dependence of water absorption and desorption in the coating film and tried to prepare test conditions that would make the water absorption/desorption behavior of the coating film in CCT consistent with that in the outdoor environments. First, we conducted absorption/desorption tests at various temperatures. In these tests, the coating films absorbed water and were then placed in a temperature and humidity controlled chamber and left to dry. The speeds of water absorption/desorption were obtained, and then the diffusion coefficients of water in the coating films at each temperature were obtained. Next, their temperature dependence (activation energy) was calculated using the diffusion coefficient at each temperature. With these values, it is possible to determine the water absorption/desorption behavior of the coating at any temperature and elapsed time. 4.2 Salt fog and humidity stepsNext, we used actual weather data to determine the temperature and time of the salt fog and humidity steps in the CCT. The coating film absorbs the maximum amount of water in the actual outdoor environment when it has been wet for a long time during a high-temperature period. The weather data indicated that the continuous wetting time (humidity of 80% RH (relative humidity) or more or precipitation) in the actual outdoor environment accounted for 20 to 50 hours, and it rarely exceeded 50 hours. It was also found that the temperature in a longer continuous wetting period was around 25°C in the summer. Therefore, we prepared a new test condition that simulates water absorption at 25°C for 50 hours. Using the temperature dependence of water absorption obtained from the above experiment, we found that the coatings absorbed the same degree of water through the salt fog step at 35°C for 0.8 hours and the humidity step at 50°C for 3 hours [2]. In contrast, the calculation of the water absorption behavior in existing CCTs (JIS K 5600-7-9 methods A and D) suggested that method A (CCT-A) was able to reproduce water absorption similar to that in the outdoor environment (25°C for 50 hours) and that method D (CCT-D) was not able to do so (Fig. 3). Although CCT-D is generally used in the evaluation of coatings for steel structures, it is not possible to reproduce water absorption that occurs in wetting for a long time during a high-temperature period. Therefore, a remaining concern was that even if coatings presented good results in CCT-D, not all of them would show good performance in actual outdoor environments.
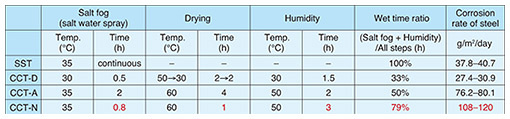
4.3 Drying stepNext, we examined the time taken for drying in the CCT. As described earlier, since corrosion does not proceed after a sample is completely dry, a shorter drying time is advantageous for shortening the test time. However, if the coating films are not sufficiently dry and tests are conducted in which the moisture content of the coating films is always high, the performance of coatings evaluated by CCT will not match the performance of coatings in actual outdoor environments. Therefore, it is necessary to ensure that the coating is sufficiently dry. Our simulations of the water desorption behavior of the coating film revealed that the coating film can dry sufficiently even if the drying time is shorter than that in existing CCTs. We therefore determined that the drying step time should be one hour. In general, the diffusion coefficient of water in polymer materials is the same for both water absorption and desorption, and in many cases, only one of them is measured. As easily imagined, it is quite difficult to reduce the time for the drying step using the same diffusion coefficient. We have already reported experimental data suggesting that the coating film had difficulty absorbing water and dried easily [3]. Therefore, we were able to carry out simulations using accurate diffusion coefficients for water absorption and desorption, which differ depending on each process, and to significantly shorten the drying step time as a result. Under the new CCT conditions in CCT-N determined through our investigations, the corrosion rate of steel was around 1.4 times higher than in CCT-A and 4 times higher than in CCT-D (Table 1).
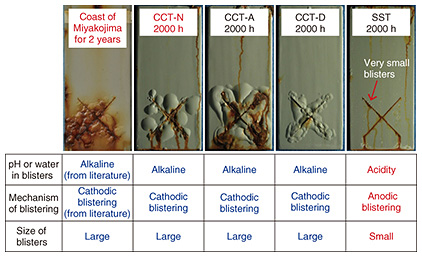
In addition, we prepared coated steel samples and exposed the samples to the outdoors in order to compare their corrosion behavior in different tests. The samples were placed on the coast of Miyakojima island, Okinawa, Japan, for two years, where CCT-N and other accelerated corrosion tests were performed. We found that the corrosion behavior in the SST without the drying step greatly differed from the behavior in the outdoor exposure, that the corrosion behavior did not differ much among various CCTs, and that CCT-N was able to reproduce the corrosion behavior of outdoor exposure well (Fig. 4) [4]. Thus, we concluded that CCT-N is an excellent accelerated corrosion test that can speed up the corrosion of steel 1.4 to 4 times compared to existing CCTs and can concurrently reproduce corrosion similar to that in the actual outdoor environment.
5. Future prospectsCCT-N can be carried out by installing test programs in common CCT instruments. Therefore, users of CCT instruments can introduce CCT-N without any additional cost. CCT-N will enable users to accurately select coatings that are more resistant to corrosion and have a longer life, in a shorter test time. Use of the appropriate selected coatings is expected to reduce the maintenance costs of steel structures. References
|
|||||||||||