|
|||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
|
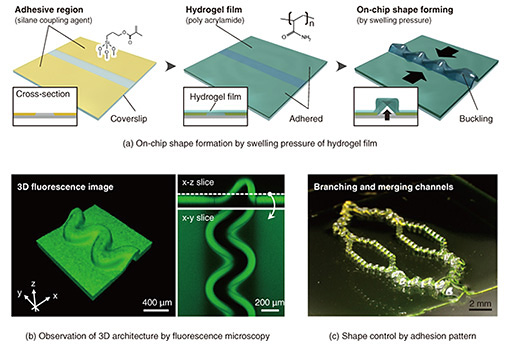
Feature Articles: Recent Updates on Bio-soft Materials Research Vol. 22, No. 5, pp. 24–30, May 2024. https://doi.org/10.53829/ntr202405fa2 Creation of Hydrogel Actuator toward Construction of On-chip Biological ModelsAbstractTo express and maintain organ-like higher-order functions in cultured cells, it is important to create an environment that is close to that of a living organism. NTT Basic Research Laboratories has established a technology to control the shape of hydrogel, a material with properties similar to those of living tissue, on a chip into forming thin-film and tubular architectures that resemble living organs. On the basis of the above-mentioned technology, this article introduces the development of an actuator that can move large, fast, and arbitrary areas by optical stimulation and a technology to control its motion as if it were a living organ. Keywords: hydrogel, actuator, organ-on-a-chip 1. Reproduction of in vivo-like environment on a chipAssay platforms*1 using cultured cells have attracted attention as an alternative to animal testing in the development of cosmetics, pharmaceuticals, agrochemicals, and other products. The use of human cells is expected to be an attractive tool that does not require consideration of species-specific differences in response, not to mention the elimination of animal ethics issues. However, to conduct appropriate evaluation without the use of living organisms, it is necessary to reproduce in vivo functions that cannot be achieved with conventional culture methods. Therefore, microphysiological systems have been extensively studied such as organ-on-a-chip technology for artificially reproducing conditions similar to those in vivo and allows cultured cells to express and maintain advanced organ functions [1]. If we can construct an on-chip biological model that reproduces organ functions on a sensor substrate based on these technologies, it is expected to be possible to acquire a variety of information on various organs as detailed data with cellular-level resolution. In addition, the use of induced pluripotent stem (iPS) cells*2 has unlimited potential in acquiring and analyzing biological data reflecting racial differences and individual genome diversity*3 at the molecular, tissue, and organ levels. To construct this on-chip biological model, the challenge is how to make the environment in which cells are cultured as close to that of in vivo as possible. For this purpose, technologies that can simultaneously help achieve bio-friendly materials, a shape close to that of the living tissue, and an in vivo stimulation environment are required. Therefore, NTT Basic Research Laboratories has focused on hydrogel, a material with high biocompatibility, and has been developing a technology to control the material’s three-dimensional (3D) shape to mimic that of a living tissue. Hydrogel is a jelly-like material in which a large amount of water is trapped in a polymer network and used in contact lenses, diapers, and other products around us. In our bodies, there are also network structures of biological macromolecules and proteins and they retain water. In other words, our body is also a hydrogel and one of the most suitable materials as a scaffold for cell growth. However, the volume of the hydrogel changes depending on the amount of water in the network, and the material is very soft and brittle due to high water content, making it difficult to control its shape on the substrate. Therefore, we reported an on-chip shape-control technology that uses the force of hydrogel to swell by absorbing water to form a thin and hollow tubular shape like a living organ*4 such as a blood vessel or intestinal tube [2]. An overview of this technology is shown in Fig. 1. According to the fabrication scheme shown in Fig. 1(a), the surface of a glass substrate is first treated with a silane coupling agent that chemically bonds to the hydrogel, and a treated/untreated pattern is formed using general lithography techniques*5 (a rectangular pattern is shown in the example). The hydrogel thin film is then synthesized on the substrate, resulting in a hydrogel thin film bonded to the glass substrate in a patterned manner. Subsequently, the hydrogel is immersed in water, which absorbs water and generates pressure due to swelling. As a result, a physical phenomenon called buckle-delamination*6 occurs in the non-adhered area to release pressure from the surroundings, forming a thin-film/tubular channel structure consisting of a hydrogel thin film and glass substrate. Figure 1(b) shows the result from fluorescent observation of a 3D structure. The obtained structure can be controlled with physical parameters, such as hydrogel hardness, thickness, water content, and geometric pattern of the non-adhered area [3], and can reproduce smooth 3D structures such as folds and wrinkles observed in vivo. Characteristic channel-like structures can also be freely designed by controlling the adhesion pattern, making it possible to fabricate branching and merging channels that mimic a vascular network (Fig. 1(c)).
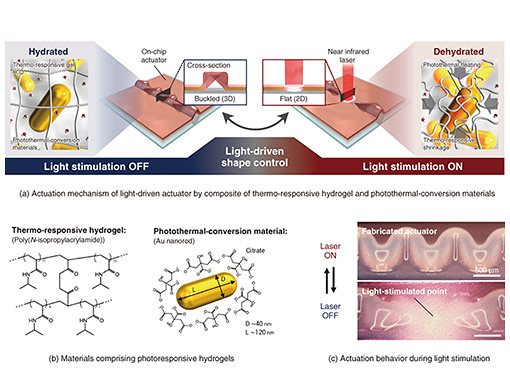
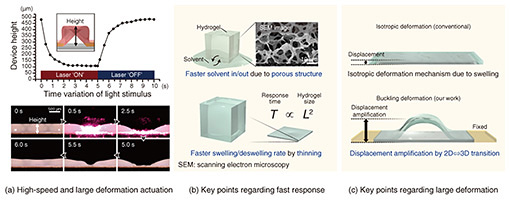
2. Hydrogel actuator with both large deformation and fast responseThe technologies introduced thus far have enabled us to fabricate a shape similar to that of a living organism on a chip using bio-friendly materials. However, to reproduce the dynamic stimulation environment similar to in vivo, it is necessary to have a technology that can “move” the shape of the hydrogel thin film. A simple method is to connect the channel structure made of the hydrogel film to an external pump to control the flow pressure. The extremely soft structure of this channel can deform significantly in response to pressure, making it possible to reproduce the pulsating contraction motion of blood vessels [4]. However, there are problems with this method, such as uniform deformation of the entire structure and the need to connect an external device, so a method for easily reproducing complex movements similar to those of a living organ is required. Therefore, we have designed and fabricated a hydrogel thin film that can be deformed by light stimulation, then proposed a light-driven actuator*7 that adapts to on-chip shape-control technology [5]. The device fabricated in this study combines a temperature-responsive hydrogel that expels water and contracts in response to temperature and a photothermal-conversion material that generates heat upon light irradiation, enabling the actuator to switch between a hydrated and dehydrated state by turning light stimulation on and off (Fig. 2(a)). The temperature-responsive hydrogel is a Polyisopropylacrylamide gel*8, which is also used as a base material for cell culture, and the photothermal-conversion material is a gold nanorod*9 that efficiently generates heat when irradiated with near-infrared light (Fig. 2(b)). Therefore, by irradiating the buckling structure of the hydrogel thin film on the chip with a near-infrared laser, it is possible to control the shape of the local buckling state and flat state. The actuation behavior is shown in Fig. 2(c). When the 3D tubular structure in the buckled state at the center of the image was irradiated with a near-infrared laser, it immediately deformed to a flat state with a completely collapsed tube, and rapidly recovered to its original buckled state again when the optical stimulation was stopped. To investigate this deformation behavior in more detail, we observed the actuator from the side and plotted the change in height of the actuator over time during light stimulation, as shown in Fig. 3(a). Surprisingly, the actuator shifted to a flat state within 2 seconds after the start of light stimulation and recovered to a buckled shape within about 2 seconds after the end of light stimulation. In addition, the actuation-deformation ratio (the ratio of the amount of deformation estimated from the maximum and minimum heights) of the actuator reached approximately 360%. This high-speed response and large deformation actuation is much higher than that of conventional light-driven hydrogel actuators.
The following is a summary of the key points of fast response and large deformation. Figure 3(b) shows the structure that enabled fast response. Conventional hydrogels have a network structure with a mesh size of several 10 nm, and water molecules move through these holes. The hydrogel used in this study has a special fabrication method that produces a network of pores several micrometers in diameter (porous structure). In other words, the hydrogel has a structure with pores nearly 1000 times larger than those of conventional hydrogels, allowing water to flow in and out easily like a sponge, and the response to volume change accompanying solvent transfer is faster. It is also known that the response time increases in proportion to the square of the hydrogel size. We used the hydrogel thin film of about 100 μm as the actuator, which enabled swelling and de-swelling in a shorter time than thicker hydrogels. Figure 3(c) shows the point of deformation that enables large deformation. When hydrogel swells by absorbing water or shrinks by expelling water, the deformation is typically isotropic, with all directions changing at the same ratio. We applied on-chip shape-control technology with which a portion of the hydrogel is fixed (restrained) to the substrate during swelling, resulting in 3D deformation due to buckling in the film-thickness direction. This deformation is large in the film thickness direction, acting as a displacement-amplification mechanism. This allows displacement that is approximately 10 times larger than the deformation ratio for each direction of normal swelling deformation, enabling large deformation.
This actuator is able to operate stably in air. Normally, actuators based on the swelling/de-swelling of hydrogel could only operate in the presence of water, making it difficult to use them in air for long periods. However, our actuator with a channel structure can hold water in the tube, allowing stable operation in air without drying out. It is also known that the actuation performance can be finely tuned by changing the hardness of the gel and height of the actuator. Therefore, as an on-chip actuator that can operate in various environments, it is expected to be applied in a wide range of fields, such as microfluidics*10 and soft robotics*11.
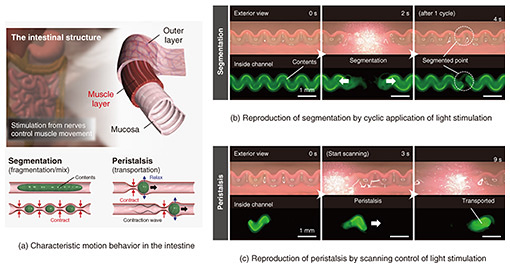
3. Demonstration of biomimetic actuationOur actuator is superior not only in performance but also in behavior control. Because the stimulus source is light, it can be remotely irradiated to the targeted position, and there is no need to connect any external devices, such as wiring, to the actuator. The combination of materials we used shrinks rapidly when heated to 35ºC or higher. By adjusting the concentration of gold nanorods, a photothermal-conversion material, the actuator is designed to heat quickly to about 35ºC during light stimulation without excessive heating. Therefore, only the irradiated area is deformed during light stimulation, and the effect of thermal diffusion on the surrounding area is suppressed. As described above, this actuator enables precise positional and local control of deformation by light stimulation, and control of complex behaviors that mimic those of biological organs is also possible. We focused on the characteristic movement of the intestinal tract, an overview of which is shown in Fig. 4(a). The intestinal tract has a three-layered structure, consisting of an outer layer that protects organs from friction, a muscular layer that generates characteristic movements, and a mucosa that handles digestion and absorption. The two characteristic movements are controlled by the fine control of the contractile movements of the muscular layer by stimulation from nerves. Specifically, the cyclic movements of contraction and relaxation are repeated at specific points to generate segmental movements that break up and agitate the intestinal contents. The contraction and relaxation propagate as a continuous wave (contraction wave) at neighboring points, which generates peristalsis that transports the intestinal contents as if whipped cream is being squeezed out. We attempted to reproduce these two types of movements by temporally and spatially controlling light stimulation (Fig. 4(b)). We first injected a highly viscous oil (mineral oil), which mimics the contents of the intestine, into the actuator. The oil was colored with a green fluorescent substance so that the contents could be observed with a fluorescence microscope. The irradiation was then repeated at specific points on the actuator in 2-second cycles to switch between buckling and flat states, i.e., open/close motion of the channel structure. It was confirmed that the continuous contents were crushed and could be separated. This is because the channel structure can be completely closed, demonstrating the possibility of reproducing segmental movement by taking advantage of the hollow structure and large deformation capability of this actuator.
We then injected a small mass of the intestinal contents model described above inside the actuator. A point slightly behind the mass was then optically stimulated to collapse the channel structure, and the irradiated point was slowly shifted (scanned). We were able to reproduce the contraction wave of the collapsed channel as it moved and confirm the movement of the contents along with it. This is due to the large deformation that can close the channel structure and the positional and local control of the deformation, demonstrating that peristaltic motion can be reproduced. Thus, it was found that complex movements of a living organ can be reproduced by using an actuator as a muscle layer and optical stimulation control as a neural control in a living organism. The motion reproduced by the actuator is comparable to that of the intestinal tract of a rat, which is a living organ of the same scale. 4. Outlook for the futureWe introduced an actuator that reproduces the in vivo stimulation environment by controlling bio-friendly materials to a shape similar to that of a living tissue as a fundamental technology for the fabricating on-chip biological models. It has become clear that physical stimuli (mechanical stress) inside and outside cells are involved in the regulation of various functions such as cell growth, differentiation, proliferation, and morphogenesis. Therefore, this technology, which can reproduce complex and dynamic mechanical stimuli of living organs on a chip, is expected to provide an in vivo-like environment and, in combination with cell culture, to provide a more sophisticated biological model. The actuator is designed to be efficiently driven at 35°C in air, which is different from the driving conditions suitable for cell culture (37°C in culture medium), but we confirmed that the operating temperature can be finely tuned by adjusting the material composition. In the future, we will select cultured cells on which we wish to examine the effects of mechanical stress and construct an experimental system in which the cells can be cultured during the application of stimuli. Integration with biosensors for acquiring biological data is also a future challenge. The actuator consists of a thin hydrogel film that can mimic the in vivo environment and a support substrate made of a solid, such as glass, in a heterogeneous (two different types) structure. The support-substrate part, from which various material types can be selected, is easy to process unlike the hydrogel thin film part. Therefore, we believe that sensors can be fabricated and integrated using semiconductor processing technology, which is NTT laboratories’ strength. If these technologies mature and can reproduce various organ functions of an individual on a high-performance biosensor substrate, it will be possible to acquire personalized biological data with high accuracy and from multiple angles. This is thought to lead to the construction of a bio-digital twin*12, a model that reproduces oneself in a digital space. In particular, the ability to acquire data under conditions that are difficult to test in actual living organisms (extreme environments, drug administration, etc.) is expected to contribute to complementing the model. We will continue to accumulate fundamental technologies to construct such on-chip biological models.
References
|
|||||||||||||||||||||||||||||||||||