|
|||||||||||||||||||||||
|
|
|||||||||||||||||||||||
|
Feature Articles: Recent Updates on Bio-soft Materials Research Vol. 22, No. 5, pp. 46–51, May 2024. https://doi.org/10.53829/ntr202405fa5 Characterization of Metal Ions in Neurons Using a Superconducting Flux QubitAbstractIn the race to develop superconducting quantum computers, superconducting quantum circuit technology has made remarkable progress. The technology has also been applied to quantum sensing, especially highly sensitive magnetometers. The target of on-chip measurement by superconducting quantum sensors has thus far been limited to impurities in semiconductors. However, the range of applications is not limited to such hard materials. This article describes the detection of ferric ions in neurons with a high-sensitivity, high-spatial-resolution magnetometer using a superconducting flux qubit. Keywords: superconducting quantum circuits, quantum sensing, biosensing 1. Trace elements in living organismsIron is a trace element that plays a significant role in the body. Physiologically, it is involved in oxygen transport via hemoglobin and energy production via adenosine triphosphate*1. Pathologically, it is involved in many neurodegenerative diseases such as Alzheimer’s disease. To understand these phenomena, it is necessary to study the transport and oxidation-state changes of ferric ions.
2. Methods for studying metal ions in biological samplesMass spectrometry is a typical method for studying metal ions in biological samples. This method is quantitative and enables cell-by-cell analysis. However, in-situ observation is difficult because the analytical process involves ionization, i.e., cell disruption by inductively coupled plasma or laser irradiation. Optical methods, such as Raman spectroscopy, are also used to analyze metallic elements. They are suitable for in-situ observation and imaging of intracellular structures. However, these methods cannot detect changes in the valence of ions due to redox reactions or protein binding, so other methods are required to obtain this information. Electron spin resonance (ESR) is widely used in various fields such as physics, chemistry, biology, geosciences, pharmaceuticals, and medicine. It is also useful for analyzing metal ions in biological samples, including ion valences. The difference between metal ions can be distinguished by determining the g-factor parameter. Typically, the analysis is conducted by filling small glass tubes with several milliliters of samples. The information obtained is therefore an average of the sample. In addition, spatial resolution and sensitivity limit cell-by-cell imaging. A magnetometer based on a superconducting quantum circuit*2 has micrometer spatial resolution and can analyze materials with unpaired electrons*3 such as metal ions. The method can therefore be a useful tool for investigating metal ions in biological samples at the cellular level.
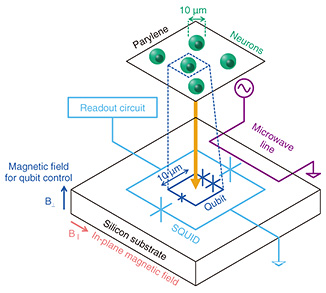
3. Quantum sensing using superconducting quantum circuitsQuantum sensing with superconducting quantum circuits is mainly aimed at ultra-sensitive measurements of magnetic fields or their source electron spins. An electron spin in materials acts as a probe for the properties of the surrounding elements. It can therefore be used as a tool for material analysis. Research on quantum sensing using superconducting quantum circuits is progressing with two main approaches. In the first approach, ultra-high sensitivity is achieved by improving the amplifier noise and other sensitivity limiting factors of ESR spectrometers using superconducting quantum circuit technology. In the second approach, small numbers of electron spins can be detected using magnetometers based on superconducting qubits, which are highly sensitive to magnetic fields. Both approaches can detect the samples in the vicinity of superconducting quantum circuit chips. NTT is researching the latter approach, using superconducting flux qubits (FQs). We developed an ultra-sensitive sensor capable of detecting 20 electron spins in 1 second of signal integration. With this sensor, we successfully analyzed solid-state samples with micrometer spatial resolution by ESR [1, 2]. The sensors based on superconducting quantum circuits can also achieve high spatial resolution because the size of the superconducting quantum sensor can be reduced. For example, an FQ consists of a rectangular loop. The size of the loop is a few micrometers on each side. It selectively detects only the magnetic field penetrating through the loop, enabling measurements with micrometer-scale spatial resolution. The high sensitivity and high spatial resolution are particularly powerful when applied to biological samples. The size of an FQ of a few micrometers is comparable to the size of a typical cell, giving us the spatial resolution to sufficiently distinguish individual cells. As the integration of superconducting qubits is progressing for quantum computing applications, it is possible to conduct cell-by-cell imaging with single-cell spatial resolution by fabricating two-dimensional arrays of FQs. As the first step toward cellular imaging, we conducted a proof-of-principle experiment to measure trace elements in cells using an FQ. We could measure ferric ions in neurons with single-cell-level spatial resolution [3]. 4. Method for detecting electron spins using an FQFigure 1 shows a schematic of the electron spin measurement system using an FQ. The FQ and neurons were placed in a dilution refrigerator, and experiments were conducted at temperatures below 200 mK (below –272.95¡î). Biological samples are rarely measured at such cryogenic temperatures. Such a combination of biological samples and low temperature raises the concern that the low temperature may alter the behavior of neurons and prevent biological information from being obtained. However, since the target of this study is the metal ions in the sample, measurements can be made even at low temperatures. In addition, the sensitivity of electron spin detection generally improves with decreasing temperature, so a low-temperature environment is preferable for measuring small amounts of metal ions.
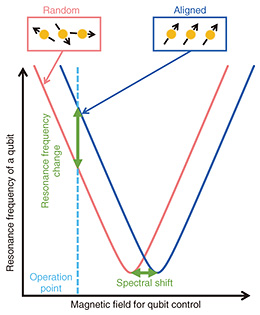
The FQ was fabricated on a silicon substrate. As shown in Fig. 1, an FQ is a loop-shaped device containing three Josephson junctions*4 (indicated by the X symbols in the figure). The clockwise and counterclockwise currents flowing through the loop correspond to two quantum states, forming a two-level quantum system. The FQ is surrounded by a superconducting quantum interference device (SQUID)*5. The SQUID is connected to an amplifier, analog-to-digital converter, personal computer, etc. at room temperature to read out the quantum state of the FQ. The transition frequency between two levels of the FQ is controlled by changing the magnetic flux through the loop. This magnetic flux is provided by a superconducting magnet placed near the FQ (B⊥ shown in Fig. 1). The principle of electron spin detection using an FQ is shown in Fig. 2. As mentioned above, FQs are usually controlled by an external superconducting magnet. However, the resonance frequency of the FQ is also changed when a sample acts as a magnetic field source in the vicinity of the FQ. If the sample is paramagnetic*6, the magnetization of the sample, i.e., the alignment of the electron spins, can be controlled by the temperature and magnetic field applied to the sample. At low temperatures and high magnetic fields, the effect of aligning electron spins along the direction of the magnetic field is greater than thermal fluctuation. Therefore, the sample shows a large magnetization (blue box in Fig. 2). In contrast, at high temperatures and low magnetic fields, the electron spins are oriented in different directions due to thermal fluctuations. Therefore, the sample shows small magnetization (red box in Fig. 2). The resonance frequency of the FQ changes because the magnetic flux through the qubit loop changes as the magnetization of the sample changes. Therefore, the magnetic properties of the sample can be studied by measuring the change in the resonance frequency of the FQ as a function of the temperature and magnetic field (B‖ in Fig. 1). It is important to note that the direction of the magnetic field for controlling the electron spins (B‖ in Fig. 1) is different from that of the magnetic field for controlling the qubit (B⊥ in Fig. 1).
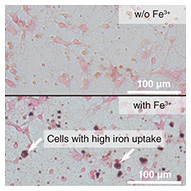
The sensitivity of electron spin detection improves when the magnetic interaction between the sample and the FQ is large. This is usually achieved by reducing the distance between them. However, since biological samples may contain liquid, insulating properties cannot be expected. Therefore, for the FQ to function properly, an insulating film must be placed between the biological sample and the FQ. We have solved this problem by using a biocompatible polymer film (parylene) with a thickness of a few micrometers for the insulation. Rat hippocampal neurons were used as biological samples. These neurons were cultured on the parylene film. To simulate neurological diseases where cells contain elevated levels of iron and other metals, the neurons were cultured in a medium supplemented with ferric ions. As shown in Fig. 3, we prepared neurons that took up more ferric ions than those cultured in a standard culture medium. The structure of neurons can be maintained at low temperatures by cross-linking proteins with paraformaldehyde and freeze-drying them after the fixation.
The parylene film with cultured neurons was placed on a silicon substrate on which the FQ had been fabricated. The loop size of the FQ is 24 × 6 μm2, about the size of a neuron. Therefore, the data obtained in the experiment can be considered to reflect the properties of a single cell.
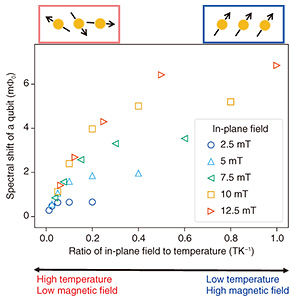
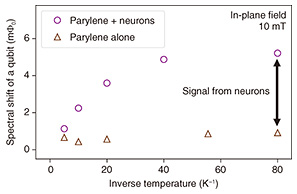
5. Measuring neurons using an FQWe first conducted an experiment to detect the magnetization of neurons. We measured the spectral shift corresponding to the change in the resonance frequency of the FQ. The in-plane magnetic field of several milli Tesla and the temperature were controlled. Figure 4 shows that at the lower temperature or higher magnetic field, the spectrum of the FQ shifted significantly: the sample showed a large magnetization. This result is consistent with the expected behavior when the sample contains paramagnetic material. Thus, the neurons and/or the parylene may contain paramagnetic components.
To determine whether the neurons or the parylene is paramagnetic, we measured only the parylene under the same experimental conditions (Fig. 5). Parylene is a polymer film composed of carbon and hydrogen that contains no unpaired electrons. In principle, it would not be paramagnetic. However, a small paramagnetic response can be observed due to damage such as oxidization. Such a control experiment is therefore necessary. Figure 5 shows that the magnetization of parylene was significantly smaller than that of neurons. This result indicates that the magnetization detected in Fig. 4 is almost entirely from the neurons.
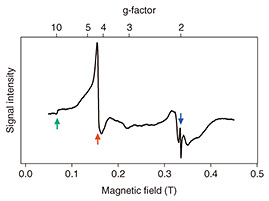
The results thus far suggest that the neurons contain some paramagnetic material. However, the origin of this paramagnetism is unknown. We collaborated with Ono and Hori laboratory at Shizuoka University, which specializes in low-temperature ESR, to further analyze the samples. The g-factor can be obtained from the ESR spectrum. By comparing this value with known material-specific g-factors, the source of the magnetization can be identified. The ESR spectrum of neurons is shown in Fig. 6. The peaks corresponding to g-factors of 9.8, 4.3, and 2.0 can be observed in this spectrum. The g-factors 9.8 and 4.3 are known to correspond to ferric ions in the cell [4]. The magnitude of the peaks indicates that this is the main source of the magnetization in the sample. Combining this result with the magnetometry result of the FQ, we can say that we have detected ferric ions in neurons with spatial resolution at the single-cell level. The peak with a g-factor of 2.0 is due to copper ions, which may also contribute to the magnetization in smaller amounts.
We conducted a qualitative analysis of metal ions in neurons using an FQ. The results of magnetometry using an FQ enable the quantification of metal ions contained in neurons. The magnitude of the change in the resonance frequency of the FQ corresponds to that of the magnetization. Therefore, this change in resonance frequency can be converted to the number of metal ions by comparing the results of a separate experiment on a reference sample with a known electron spin concentration. The results in Fig. 4 indicate that the measured rat hippocampal neurons contained 8 μg of iron per gram in the dry state. This result is consistent with 2 to 34 μg/g obtained in previous studies on human brain cells [4, 5]. 6. ProspectsUsing quantum sensing technology with FQs, we have successfully detected and analyzed metal ions in neurons quantitatively and qualitatively with single-cell-level spatial resolution. Future work includes single-cell resolution ESR spectroscopy of neurons using an FQ and imaging of metal ions in tissues by fabricating two-dimensional arrays of FQs. References
|
|||||||||||||||||||||||