|
|||||||||||||||||||
|
|
|||||||||||||||||||
|
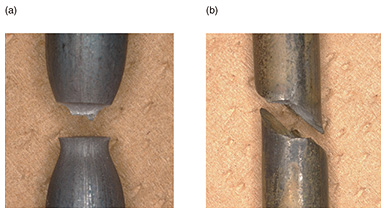
Regular Articles Vol. 22, No. 5, pp. 110–115, May 2024. https://doi.org/10.53829/ntr202405ra1 Sub-surface-hydrogen-measurement Method for Estimating Hydrogen-embrittlement Risk in Concrete PolesAbstractConcrete poles play an important role in the infrastructure providing communication services to customers. If the risk of hydrogen embrittlement of rebar embedded in concrete can be determined from information about the environment in which the equipment is installed, the safety of concrete poles can be further improved. Our research group has developed a method for measuring the sub-surface hydrogen concentration of rebar in concrete poles, which is one of the most important parameters for measuring hydrogen embrittlement. Keywords: deterioration risk evaluation, concrete poles, hydrogen embrittlement 1. Maintenance of concrete polesVarious infrastructure structures, such as bridges and steel towers, support daily life. Improving the safety of such infrastructures can make our lives sustainable and better. Our group is researching the deterioration of materials used in infrastructure to contribute to its maintenance and management from the viewpoint of materials science. Concrete poles are one of the most important components of the infrastructure providing communication and power to customers. Many are located very close to us. Therefore, if one breaks, it may lead to a serious accident or communication outages. High safety is required for concrete poles to prevent serious accidents. A concrete pole is a structure consisting of concrete and rebar, and the safety of the pole is secured by the rebar inside. The rebar is protected from corrosion by the alkaline environment of the concrete. Since rebar is embedded in concrete under tensile stress, it applies compressive stress on the concrete. This prevents the concrete from cracking. As described above, concrete and rebar complement each other in strength, so the rebar in a concrete pole does not break under normal conditions. However, when various negative conditions combine, a degradation phenomenon known as hydrogen embrittlement may develop, leading to a reduction in the strength of concrete poles [1–3]. It is difficult to detect the deterioration of rebar because it is embedded in the concrete and cannot be inspected visually. For such invisible deterioration, it is useful to use a method for evaluating the risk of deterioration from environmental information surrounding the location of the equipment, such as temperature and humidity [4]. If it is possible to evaluate the hydrogen-embrittlement risk of rebar in concrete poles from environmental information, it would be possible to preferentially update equipment that is at high risk. This could improve the safety of entire facilities. 2. Hydrogen embrittlement of rebar in concrete polesHydrogen embrittlement is a phenomenon in which the strength of a metal decreases due to hydrogen atoms entering the metal, leading to premature cracking and fracture. Whereas normal metals fracture in a ductile manner, metals degraded by hydrogen embrittlement have a glass-like fracture surface morphology (Fig. 1). This phenomenon, also known as delayed fracture, occurs decades after equipment is installed, and its progression is difficult to evaluate. The mechanism is not well understood and is being actively discussed by many researchers worldwide.
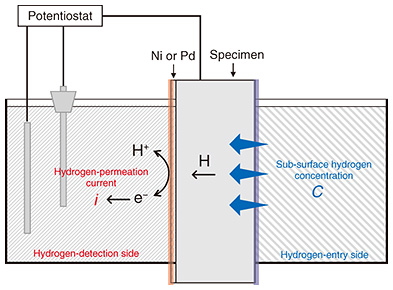
Hydrogen embrittlement is considered to develop in concrete poles as follows. Cracks first appear in concrete due to unfortunate circumstances. If a crack is large enough to reach the rebar, the alkaline concrete adhering to the rebar is locally lost around where the crack reached, i.e., the ability to inhibit corrosion is lost, so corrosion of the steel begins. Hydrogen is generated by the reduction reaction of water (cathodic reaction) that proceeds together with the dissolution reaction of iron (anodic reaction). Some of the generated hydrogen enters the rebar as hydrogen atoms, causing the rebar to undergo hydrogen embrittlement [5]. Fe → Fe2+ + 2e− (anodic reaction) 2H2O + 2e− → H2 + 2OH− (cathodic reaction) One of the most important parameters in evaluating the risk of hydrogen embrittlement is the sub-surface hydrogen concentration of rebar. The higher the amount of sub-surface hydrogen in the rebar generated by corrosion, the greater the risk of hydrogen-embrittlement fracture. It is therefore vital to know how the sub-surface hydrogen concentration of rebar in a concrete pole is affected by environmental factors, but there is currently no way to measure this. 3. Hydrogen-permeation testElectrochemical hydrogen-permeation tests are typically conducted to measure the sub-surface hydrogen concentration [6]. Hydrogen atoms entering from one side of the test object are ionized on the nickel (Ni) or palladium (Pd) passivation layer formed on the opposite side of the penetration side and detected as a hydrogen-permeation current (Fig. 2). Assuming that hydrogen diffuses one-dimensionally over a sample length, the following relationship is known between the hydrogen permeation current and sub-surface hydrogen concentration [7].
where i is the hydrogen permeation current, C is the sub-surface hydrogen concentration, F is Faraday’s constant, and L is the sample length. Therefore, the sub-surface hydrogen concentration of a sample can be determined by measuring the hydrogen-permeation current. This is a relatively simple method for measuring the sub-surface hydrogen concentration and is the only method that can measure it continuously and nondestructively. However, the general electrochemical hydrogen-permeation test is limited in its test geometry to plates only. Therefore, it is not possible to measure the sub-surface hydrogen concentration of rebar in concrete poles with such a method. We developed a hydrogen permeation test method for concrete samples cut from concrete poles, and the sub-surface hydrogen concentration of rebar in concrete poles was measured.
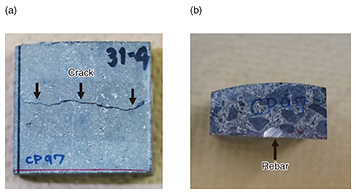
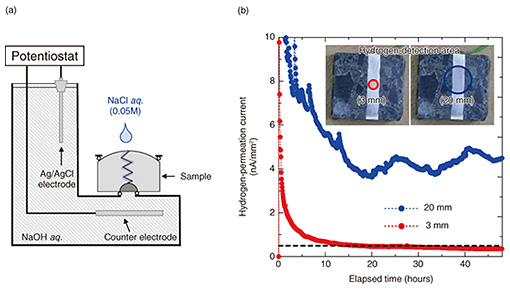
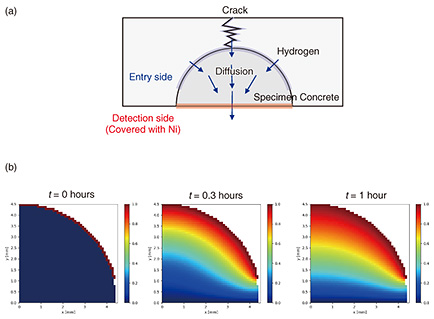
4. Hydrogen-permeation test extended to concrete specimensThe concrete sample we used for this study was about 4-cm square and included rebar cut from a concrete pole (Fig. 3). A crack was introduced into the concrete sample by using the three-point bending method. The sample was set in a self-made electrochemical test cell (L-shaped cell) and was in contact with a sodium hydroxide (NaOH) solution packed in the cell on the hydrogen-detection side (Fig. 4(a)). The hydrogen-detection area was set as a 3-mm circle to prevent the solution from contacting the concrete and allow only the rebar to contact the solution, thereby reducing the noise current (Fig. 4(b)). Hydrogen was generated by dropping 100 μL of 0.05M sodium chloride (NaCl) solution (equivalent to rainwater) into the concrete crack to cause the rebar to artificially corrode. The solution traveled through the crack and contacted the rebar, causing the corrosion reaction to proceed and start hydrogen generation. The corrosion conditions of the rebar were assumed to be those for the corrosion of rebar in concrete poles in outdoor environments. To obtain the sub-surface hydrogen concentration of rebar from the obtained experimental data, a simulation of hydrogen diffusing through rebar was carried out. The calculation was carried out assuming that atomic hydrogen diffuses in a round rod according to the following two-dimensional diffusion equation.
The corrosion reaction, i.e., hydrogen penetration, was assumed to occur on the surface where rebar was located just under the crack. A boundary condition with a constant sub-surface hydrogen concentration was set along the rebar surface, as shown in Fig. 5(a). As shown in Fig. 5(b), hydrogen penetrates from the steel surface and diffuses through the steel to reach the hydrogen detection surface. To reduce computational complexity, the simulation was conducted with a four-quarter circle in consideration of symmetry [8].
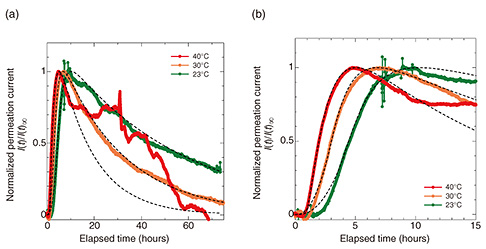
5. Hydrogen-penetration behavior of rebar in concrete polesFigure 6 shows the results for the penetration current obtained experimentally and the calculated penetration current obtained by simulation at various temperatures (23, 30, and 40°C). The results of the theoretical simulation were generally consistent with the experimental values. The change in sub-surface hydrogen concentration over time obtained from the simulation at each temperature (~0.08 ppm) was consistent with the amount of hydrogen that was supposed to enter the steel by atmospheric corrosion (0.1 ppm) [9]. Due to the crack in the concrete, oxygen diffusion was suppressed and slightly low. These results indicate that the hydrogen generated in the concrete and diffused in the round rod was correctly captured with our method.
At all temperatures, the hydrogen-permeation current began to increase a certain period (about 2 hours at 23°C) after the NaCl solution was dropped and peaked several hours later (about 10 hours at 23°C). The higher the temperature, the faster the hydrogen-permeation current increased and reached maximum. These results are reasonable considering the low diffusion coefficient of high-strength steel and the temperature dependence of the diffusion coefficient. Thereafter, a gradual downward trend was observed over 48 hours. Compared with the test results for atmospheric corrosion of bare pure iron, the decrease in the hydrogen-permeation current was extremely gradual, meaning that the corrosion continued for a long period. This long-lasting corrosion is attributed to the fact that cracks in concrete strongly retain water. It is assumed that a considerable amount of moisture is retained inside concrete cracks for a long period, and a condition such as solution corrosion is assumed to continue. The difference between the experimental and simulated results at 40°C is considered due to the rapid evaporation rate of the solution. Evaporation of the solution in the crack led to the concentration of chloride ions and accelerated corrosion. By using our hydrogen-permeation test method, it was possible to clarify the hydrogen-penetration behavior of rebar in concrete. This knowledge can be used in technology for evaluating the risk of hydrogen embrittlement of rebar in concrete poles and will be important for examining methods for repairing concrete poles. 6. ConclusionTo further improve the safety of concrete poles, it is useful to estimate the risk of hydrogen embrittlement of rebar embedded in concrete from environmental information on installed equipment. We developed a method for measuring the sub-surface hydrogen concentration of rebar in concrete poles, which is important for evaluating the risk of hydrogen embrittlement of rebar. Our method is considered important for understanding how hydrogen embrittlement of concrete poles proceeds. References
|
|||||||||||||||||||